Site-isolated redox reactivity in a trinuclear iron complex
- PMID: 22988949
- PMCID: PMC3464975
- DOI: 10.1021/ic301241s
Site-isolated redox reactivity in a trinuclear iron complex
Abstract
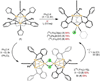
The symmetric, high-spin triiron complex ((Ph)L)Fe(3)(THF)(3) reacts with mild chemical oxidants (e.g., Ph(3)C-X, I(2)) to afford an asymmetric core, where one iron bears the halide ligand ((Ph)L)Fe(3)X(L) and the hexadentate ((Ph)L = MeC(CH(2)NPh-o-NPh)(3)) ligand has undergone significant rearrangement. In the absence of a suitable trapping ligand, the chlorine and bromine complexes form (μ-X)(2)-bridged structures of the type [((Ph)L)Fe(3)(μ-X)](2). In the trinuclear complexes, the halide-bearing iron site sits in approximate trigonal-bipyramidal (tbp) geometry, formed by two ((Ph)L) anilides and an exogenous solvent molecule. The two distal iron atoms reside in distorted square-planar sites featuring a short Fe-Fe separation at 2.301 Å, whereas the distance to the tbp site is substantially elongated (2.6-2.7 Å). Zero-field, (57)Fe Mössbauer analysis reveals the diiron unit as the locus of oxidation, while the tbp site bearing the halide ligand remains divalent. Magnetic data acquired for the series reveal that the oxidized diiron unit comprises a strongly coupled S = (3)/(2) unit that is weakly ferromagnetically coupled to the high-spin (S = 2) ferrous site, giving an overall S = (7)/(2) ground state for the trinuclear units.
Figures
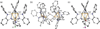

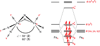
Similar articles
-
Metal atom lability in polynuclear complexes.Inorg Chem. 2013 May 6;52(9):5006-12. doi: 10.1021/ic302694y. Epub 2013 Apr 23. Inorg Chem. 2013. PMID: 23642178 Free PMC article.
-
Oxidative group transfer to a triiron complex to form a nucleophilic μ(3)-nitride, [Fe3(μ(3)-N)]-.J Am Chem Soc. 2011 Mar 16;133(10):3336-8. doi: 10.1021/ja2003445. Epub 2011 Feb 18. J Am Chem Soc. 2011. PMID: 21332160 Free PMC article.
-
Accessibility and selective stabilization of the principal spin states of iron by pyridyl versus phenolic ketimines: modulation of the 6A1 ↔ 2T2 ground-state transformation of the [FeN4O2]+ chromophore.Inorg Chem. 2012 Aug 6;51(15):8241-53. doi: 10.1021/ic300732r. Epub 2012 Jul 18. Inorg Chem. 2012. PMID: 22808945
-
Catalytic C-H bond amination from high-spin iron imido complexes.J Am Chem Soc. 2011 Apr 6;133(13):4917-23. doi: 10.1021/ja110066j. Epub 2011 Mar 15. J Am Chem Soc. 2011. PMID: 21405138
-
Iron corrolates: unambiguous chloroiron(III) (corrolate)(2-.) pi-cation radicals.J Inorg Biochem. 2006 Apr;100(4):810-37. doi: 10.1016/j.jinorgbio.2006.01.038. Epub 2006 Mar 7. J Inorg Biochem. 2006. PMID: 16519943 Review.
Cited by
-
Synthesis, structure and reactivity of μ3-SnH capped trinuclear nickel cluster.Chem Sci. 2022 Sep 5;13(38):11382-11387. doi: 10.1039/d2sc04042e. eCollection 2022 Oct 5. Chem Sci. 2022. PMID: 36320577 Free PMC article.
-
Ligand Field Strength Mediates Electron Delocalization in Octahedral [((H)L)2Fe6(L')m](n+) Clusters.J Am Chem Soc. 2015 Sep 2;137(34):11126-43. doi: 10.1021/jacs.5b06453. Epub 2015 Aug 21. J Am Chem Soc. 2015. PMID: 26231520 Free PMC article.
-
Synthesis of open-shell, bimetallic Mn/Fe trinuclear clusters.J Am Chem Soc. 2013 Sep 25;135(38):14448-58. doi: 10.1021/ja408003d. Epub 2013 Sep 11. J Am Chem Soc. 2013. PMID: 23984911 Free PMC article.
-
A Thermodynamic Model for Redox-Dependent Binding of Carbon Monoxide at Site-Differentiated, High Spin Iron Clusters.J Am Chem Soc. 2018 Apr 25;140(16):5569-5578. doi: 10.1021/jacs.8b01825. Epub 2018 Apr 12. J Am Chem Soc. 2018. PMID: 29589921 Free PMC article.
-
Dinitrogen binding and cleavage by multinuclear iron complexes.Acc Chem Res. 2015 Jul 21;48(7):2059-65. doi: 10.1021/acs.accounts.5b00213. Epub 2015 Jun 23. Acc Chem Res. 2015. PMID: 26099821 Free PMC article. Review.
References
-
-
Nitrogenase: Burgess BK, Lowe DJ. Chem. Rev. 1996;96:2983–3012. Dos Santos PC, Igarashi RY, Lee HI, Hoffman BM, Seefeldt LC, Dean DR. Acc. Chem. Res. 2005;38:208–214. Hoffman BM, Dean DR, Seefeldt LC. Acc. Chem. Res. 2009;42:609–619. Photosystem II: Nugent J, editor. Biochim. Biophys. Acta. 2001;1503:1. Ferreira KN, Iverson TM, Maghlaoui K, Barber J, Iwata S. Science. 2004;303:1831. Iwata S, Barber J. Curr. Opin. Struct. Biol. 2004;14:447. N2O reductase: Brown K, Djinovic-Carugo K, Haltia T, Cabrito I, Saraste M, Moura JJG, Moura I, Tegoni M, Cambillau C. J. Biol. Chem. 2000;275:41133. Brown K, Tegoni M, Prudêncio M, Pereira AS, Besson S, Moura JJ, Moura I, Cambillau C. Nat. Struct. Biol. 2000;7:191. Chen P, George SD, Cabrito I, Antholine WE, Moura JG, Moura I, Hedman B, Hodgson KO, Solomon EI. J. Am. Chem. Soc. 2002;124:744.
-
-
- Zhao Q, Betley TA. Angew. Chem. Int. Ed. . 2011;50:709–712. - PubMed
- Powers TM, Fout AR, Zheng SL, Betley TA. J. Am. Chem. Soc. 2011;133:3336. - PMC - PubMed
- Zhao Q, Harris TD, Betley TA. J. Am. Chem. Soc. 2011;133:8293. - PubMed
- Harris TD, Zhao Q, Hernández Sánchez R, Betley TA. Chem. Commun. 2011;47:6344. - PubMed
- Harris TD, Betley TA. J. Am. Chem. Soc. 2011;133:13852. - PubMed
- Fout AR, Zhao Q, Xiao DJ, Betley TA. J. Am. Chem. Soc. 2011;133:16750. - PMC - PubMed
- Eames EV, Harris TD, Betley TA. Chem. Sci. 2012;3:407.
-
- Adams RD. J. Organometallic Chem. 2000;600:1–6.
- Suzuki H. Eur. J. Inorg. Chem. 2002:1009–1023.
- Dyson PJ. Coord. Chem. Rev. 2004;248:2443–2458.
- Pap JS, DeBeer George S, Berry JF. Angew. Chem. Int. Ed. 2008;47:10102–10105. - PubMed
-
- Gomberg M. J. Am. Chem. Soc. 1900;22:757.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

