Expression, purification, and reconstitution of the voltage-sensing domain from Ci-VSP
- PMID: 22989304
- PMCID: PMC3845960
- DOI: 10.1021/bi300980q
Expression, purification, and reconstitution of the voltage-sensing domain from Ci-VSP
Abstract
The voltage-sensing domain (VSD) is the common scaffold responsible for the functional behavior of voltage-gated ion channels, voltage sensitive enzymes, and proton channels. Because of the position of the voltage dependence of the available VSD structures, at present, they all represent the activated state of the sensor. Yet in the absence of a consensus resting state structure, the mechanistic details of voltage sensing remain controversial. The voltage dependence of the VSD from Ci-VSP (Ci-VSD) is dramatically right shifted, so that at 0 mV it presumably populates the putative resting state. Appropriate biochemical methods are an essential prerequisite for generating sufficient amounts of Ci-VSD protein for high-resolution structural studies. Here, we present a simple and robust protocol for the expression of eukaryotic Ci-VSD in Escherichia coli at milligram levels. The protein is pure, homogeneous, monodisperse, and well-folded after solubilization in Anzergent 3-14 at the analyzed concentration (~0.3 mg/mL). Ci-VSD can be reconstituted into liposomes of various compositions, and initial site-directed spin labeling and electron paramagnetic resonance (EPR) spectroscopic measurements indicate its first transmembrane segment folds into an α-helix, in agreement with the homologous region of other VSDs. On the basis of our results and enhanced relaxation EPR spectroscopy measurement, Ci-VSD reconstitutes essentially randomly in proteoliposomes, precluding straightforward application of transmembrane voltages in combination with spectroscopic methods. Nevertheless, these results represent an initial step that makes the resting state of a VSD accessible to a variety of biophysical and structural approaches, including X-ray crystallography, spectroscopic methods, and electrophysiology in lipid bilayers.
Figures

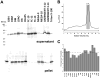
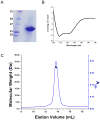

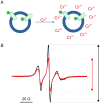
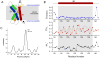
Similar articles
-
Transfer of Kv3.1 voltage sensor features to the isolated Ci-VSP voltage-sensing domain.Biophys J. 2012 Aug 22;103(4):669-76. doi: 10.1016/j.bpj.2012.07.031. Biophys J. 2012. PMID: 22947928 Free PMC article.
-
Sensing charges of the Ciona intestinalis voltage-sensing phosphatase.J Gen Physiol. 2013 Nov;142(5):543-55. doi: 10.1085/jgp.201310993. Epub 2013 Oct 14. J Gen Physiol. 2013. PMID: 24127524 Free PMC article.
-
Tuning the voltage-sensor motion with a single residue.Biophys J. 2012 Aug 8;103(3):L23-L25. doi: 10.1016/j.bpj.2012.06.030. Biophys J. 2012. PMID: 22947880 Free PMC article.
-
Biodiversity of voltage sensor domain proteins.Pflugers Arch. 2007 Jun;454(3):361-71. doi: 10.1007/s00424-007-0222-6. Epub 2007 Mar 9. Pflugers Arch. 2007. PMID: 17347852 Review.
-
New insights in the activity of voltage sensitive phosphatases.Cell Signal. 2012 Aug;24(8):1541-7. doi: 10.1016/j.cellsig.2012.03.013. Epub 2012 Mar 28. Cell Signal. 2012. PMID: 22481094 Review.
Cited by
-
Purification and structural study of the voltage-sensor domain of the human KCNQ1 potassium ion channel.Biochemistry. 2014 Apr 1;53(12):2032-42. doi: 10.1021/bi500102w. Epub 2014 Mar 18. Biochemistry. 2014. PMID: 24606221 Free PMC article.
-
Conduits of life's spark: a perspective on ion channel research since the birth of neuron.Neuron. 2013 Oct 30;80(3):658-74. doi: 10.1016/j.neuron.2013.10.040. Neuron. 2013. PMID: 24183018 Free PMC article. Review.
-
Detergent screening of the human voltage-gated proton channel using fluorescence-detection size-exclusion chromatography.Protein Sci. 2014 Aug;23(8):1136-47. doi: 10.1002/pro.2492. Epub 2014 Jun 14. Protein Sci. 2014. PMID: 24863684 Free PMC article.
-
Fifty years of gating currents and channel gating.J Gen Physiol. 2023 Aug 7;155(8):e202313380. doi: 10.1085/jgp.202313380. Epub 2023 Jul 6. J Gen Physiol. 2023. PMID: 37410612 Free PMC article. Review.
-
Biochemical and structural analysis of the hyperpolarization-activated K(+) channel MVP.Biochemistry. 2014 Mar 18;53(10):1627-36. doi: 10.1021/bi4014243. Epub 2014 Mar 7. Biochemistry. 2014. PMID: 24490868 Free PMC article.
References
-
- Bezanilla F. How membrane proteins sense voltage. Nat Rev Mol Cell Biol. 2008;9:323–332. - PubMed
-
- Murata Y, Iwasaki H, Sasaki M, Inaba K, Okamura Y. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature. 2005;435:1239–1243. - PubMed
-
- Sasaki M, Takagi M, Okamura Y. A voltage sensor-domain protein is a voltage-gated proton channel. Science. 2006;312:589–592. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

