Nanoemulsion W805EC improves immune responses upon intranasal delivery of an inactivated pandemic H1N1 influenza vaccine
- PMID: 22989689
- PMCID: PMC3488283
- DOI: 10.1016/j.vaccine.2012.09.007
Nanoemulsion W805EC improves immune responses upon intranasal delivery of an inactivated pandemic H1N1 influenza vaccine
Abstract
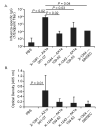
Currently available influenza vaccines provide suboptimal protection. In order to improve the quality of protective immune responses elicited following vaccination, we developed an oil-in-water nanoemulsion (NE)-based adjuvant for an intranasally-delivered inactivated influenza vaccine. Using a prime-boost vaccination regimen, we show that intranasal vaccines containing the W(80)5EC NE elicited higher titers of serum hemagglutination inhibiting (HAI) antibody and influenza-specific IgG and IgA titers compared to vaccines that did not contain the NE. Similarly, vaccines containing the W(80)5EC NE resulted in higher influenza-specific IgA levels in the bronchoalveolar lavage (BAL) fluid and nasal wash when compared to vaccines formulated without NE. The higher antibody titers in mice immunized with the NE-containing vaccines correlated with reduced viral loads in the lungs and nasal turbinates following a high dose viral challenge. Mice immunized with vaccines containing the W(80)5EC NE also showed a reduction in body weight loss following challenge compared to mice immunized with equivalent vaccines produced without NE. Taken together, our results show that the W(80)5EC NE substantially improves the magnitude of protective influenza-specific antibody responses and is a promising mucosal adjuvant for influenza vaccines and vaccines against other mucosal pathogens.
Published by Elsevier Ltd.
Figures

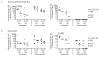


References
-
- Glezen WP. Cold-adapted, live attenuated influenza vaccine. Expert Rev Vaccines. 2004;3(2):131–9. - PubMed
-
- Riddiough MA, Sisk JE, Bell JC. Influenza vaccination. JAMA. 1983;249(23):3189–95. - PubMed
-
- Ghendon Y. The immune response of humans to live and inactivated influenza vaccines. Adv Exp Med Biol. 1989;257:37–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

