The roles of WRN and BLM RecQ helicases in the Alternative Lengthening of Telomeres
- PMID: 22989712
- PMCID: PMC3510502
- DOI: 10.1093/nar/gks862
The roles of WRN and BLM RecQ helicases in the Alternative Lengthening of Telomeres
Abstract
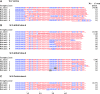
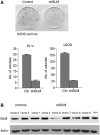
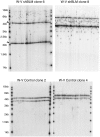
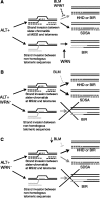
Approximately 10% of all cancers, but a higher proportion of sarcomas, use the recombination-based alternative lengthening of telomeres (ALT) to maintain telomeres. Two RecQ helicase genes, BLM and WRN, play important roles in homologous recombination repair and they have been implicated in telomeric recombination activity, but their precise roles in ALT are unclear. Using analysis of sequence variation present in human telomeres, we found that a WRN- ALT+ cell line lacks the class of complex telomere mutations attributed to inter-telomeric recombination in other ALT+ cell lines. This suggests that WRN facilitates inter-telomeric recombination when there are sequence differences between the donor and recipient molecules or that sister-telomere interactions are suppressed in the presence of WRN and this promotes inter-telomeric recombination. Depleting BLM in the WRN- ALT+ cell line increased the mutation frequency at telomeres and at the MS32 minisatellite, which is a marker of ALT. The absence of complex telomere mutations persisted in BLM-depleted clones, and there was a clear increase in sequence homogenization across the telomere and MS32 repeat arrays. These data indicate that BLM suppresses unequal sister chromatid interactions that result in excessive homogenization at MS32 and at telomeres in ALT+ cells.
Figures

Similar articles
-
WRN loss induces switching of telomerase-independent mechanisms of telomere elongation.PLoS One. 2014 Apr 7;9(4):e93991. doi: 10.1371/journal.pone.0093991. eCollection 2014. PLoS One. 2014. PMID: 24709898 Free PMC article.
-
Human RECQL1 participates in telomere maintenance.Nucleic Acids Res. 2014 May;42(9):5671-88. doi: 10.1093/nar/gku200. Epub 2014 Mar 12. Nucleic Acids Res. 2014. PMID: 24623817 Free PMC article.
-
POT1 stimulates RecQ helicases WRN and BLM to unwind telomeric DNA substrates.J Biol Chem. 2005 Sep 16;280(37):32069-80. doi: 10.1074/jbc.M505211200. Epub 2005 Jul 18. J Biol Chem. 2005. PMID: 16030011
-
Unwinding protein complexes in ALTernative telomere maintenance.J Cell Biochem. 2010 Jan 1;109(1):7-15. doi: 10.1002/jcb.22388. J Cell Biochem. 2010. PMID: 19911388 Free PMC article. Review.
-
Roles of the Werner syndrome RecQ helicase in DNA replication.DNA Repair (Amst). 2008 Nov 1;7(11):1776-86. doi: 10.1016/j.dnarep.2008.07.017. Epub 2008 Sep 6. DNA Repair (Amst). 2008. PMID: 18722555 Free PMC article. Review.
Cited by
-
WRN loss induces switching of telomerase-independent mechanisms of telomere elongation.PLoS One. 2014 Apr 7;9(4):e93991. doi: 10.1371/journal.pone.0093991. eCollection 2014. PLoS One. 2014. PMID: 24709898 Free PMC article.
-
Functional impairment of bone formation in the pathogenesis of osteoporosis: the bone marrow regenerative competence.Curr Osteoporos Rep. 2013 Jun;11(2):117-25. doi: 10.1007/s11914-013-0139-2. Curr Osteoporos Rep. 2013. PMID: 23471774 Review.
-
The absence of (TCAGGG)n repeats in some telomeres, combined with variable responses to NR2F2 depletion, suggest that this nuclear receptor plays an indirect role in the alternative lengthening of telomeres.Sci Rep. 2020 Nov 26;10(1):20597. doi: 10.1038/s41598-020-77606-w. Sci Rep. 2020. PMID: 33244044 Free PMC article.
-
A high-throughput screen to identify novel small molecule inhibitors of the Werner Syndrome Helicase-Nuclease (WRN).PLoS One. 2019 Jan 9;14(1):e0210525. doi: 10.1371/journal.pone.0210525. eCollection 2019. PLoS One. 2019. PMID: 30625228 Free PMC article.
-
Human RECQL1 participates in telomere maintenance.Nucleic Acids Res. 2014 May;42(9):5671-88. doi: 10.1093/nar/gku200. Epub 2014 Mar 12. Nucleic Acids Res. 2014. PMID: 24623817 Free PMC article.
References
-
- Chu WK, Hickson ID. RecQ helicases: multifunctional genome caretakers. Nat. Rev. Cancer. 2009;9:644–654. - PubMed
-
- Chu WK, Hanada K, Kanaar R, Hickson ID. BLM has early and late functions in homologous recombination repair in mouse embryonic stem cells. Oncogene. 2010;29:4705–4714. - PubMed
-
- van Brabant AJ, Ye T, Sanz M, German IJ, Ellis NA, Holloman WK. Binding and melting of D-loops by the Bloom syndrome helicase. Biochemistry. 2000;39:14617–14625. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

