Global transcriptional control by glucose and carbon regulator CcpA in Clostridium difficile
- PMID: 22989714
- PMCID: PMC3510511
- DOI: 10.1093/nar/gks864
Global transcriptional control by glucose and carbon regulator CcpA in Clostridium difficile
Abstract
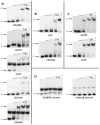
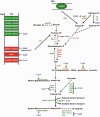
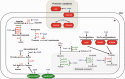
The catabolite control protein CcpA is a pleiotropic regulator that mediates the global transcriptional response to rapidly catabolizable carbohydrates, like glucose in Gram-positive bacteria. By whole transcriptome analyses, we characterized glucose-dependent and CcpA-dependent gene regulation in Clostridium difficile. About 18% of all C. difficile genes are regulated by glucose, for which 50% depend on CcpA for regulation. The CcpA regulon comprises genes involved in sugar uptake, fermentation and amino acids metabolism, confirming the role of CcpA as a link between carbon and nitrogen pathways. Using combination of chromatin immunoprecipitation and genome sequence analysis, we detected 55 CcpA binding sites corresponding to ∼140 genes directly controlled by CcpA. We defined the C. difficile CcpA consensus binding site (cre(CD) motif), that is, 'RRGAAAANGTTTTCWW'. Binding of purified CcpA protein to 19 target cre(CD) sites was demonstrated by electrophoretic mobility shift assay. CcpA also directly represses key factors in early steps of sporulation (Spo0A and SigF). Furthermore, the C. difficile toxin genes (tcdA and tcdB) and their regulators (tcdR and tcdC) are direct CcpA targets. Finally, CcpA controls a complex and extended regulatory network through the modulation of a large set of regulators.
Figures





References
-
- Ricciardi R, Rothenberger DA, Madoff RD, Baxter NN. Increasing prevalence and severity of Clostridium difficile colitis in hospitalized patients in the United States. Arch. Surg. 2007;142:624–631; discussion 631. - PubMed
-
- Loo VG, Poirier L, Miller MA, Oughton M, Libman MD, Michaud S, Bourgault AM, Nguyen T, Frenette C, Kelly M, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N. Engl. J. Med. 2005;353:2442–2449. - PubMed
-
- Bartlett JG. Narrative review: the new epidemic of Clostridium difficile-associated enteric disease. Ann. Intern. Med. 2006;145:758–764. - PubMed
-
- Akerlund T, Svenungsson B, Lagergren A, Burman LG. Correlation of disease severity with fecal toxin levels in patients with Clostridium difficile-associated diarrhea and distribution of PCR ribotypes and toxin yields in vitro of corresponding isolates. J. Clin. Microbiol. 2006;44:353–358. - PMC - PubMed
-
- Deneve C, Janoir C, Poilane I, Fantinato C, Collignon A. New trends in Clostridium difficile virulence and pathogenesis. Int. J. Antimicrob. Agents. 2009;33(Suppl. 1):S24–S28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

