Recombinant expression of TLR5 proteins by ligand supplementation and a leucine-rich repeat hybrid technique
- PMID: 22989748
- PMCID: PMC3823237
- DOI: 10.1016/j.bbrc.2012.09.021
Recombinant expression of TLR5 proteins by ligand supplementation and a leucine-rich repeat hybrid technique
Abstract
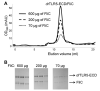
Vertebrate TLR5 directly binds bacterial flagellin proteins and activates innate immune responses against pathogenic flagellated bacteria. Structural and biochemical studies on the TLR5/flagellin interaction have been challenging due to the technical difficulty in obtaining active recombinant proteins of TLR5 ectodomain (TLR5-ECD). We recently succeeded in production of the N-terminal leucine rich repeats (LRRs) of Danio rerio (dr) TLR5-ECD in a hybrid with another LRR protein, hagfish variable lymphocyte receptor (VLR), and determined the crystal structure of its complex with flagellin D1-D2-D3 domains. Although the structure provides valuable information about the interaction, it remains to be revealed how the C-terminal region of TLR5-ECD contributes to the interaction. Here, we present two methods to obtain recombinant TLR5 proteins that contain the C-terminal region in a baculovirus expression system. First, production of biologically active full-length drTLR5-ECD was substantially enhanced by supplementation of expression culture with purified flagellin proteins. Second, we designed TLR5-VLR hybrids using an LRR hybrid technology by single and double LRR fusions and were able to express diverse regions of drTLR5-ECD, allowing us to detect a previously unidentified TLR5/flagellin interaction. The drTLR5-VLR hybrid technique was also successfully applied to human TLR5-ECD whose expression has been highly problematic. These alternative TLR5 expression strategies provide an opportunity to obtain a complete view of the TLR5/flagellin interaction and can be applied to other LRR proteins.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures


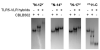
References
-
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu. Rev. Immunol. 2003;21:335–376. - PubMed
-
- Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, Aderem A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

