Domain organization of the polymerizing mannosyltransferases involved in synthesis of the Escherichia coli O8 and O9a lipopolysaccharide O-antigens
- PMID: 22989876
- PMCID: PMC3488083
- DOI: 10.1074/jbc.M112.412577
Domain organization of the polymerizing mannosyltransferases involved in synthesis of the Escherichia coli O8 and O9a lipopolysaccharide O-antigens
Abstract
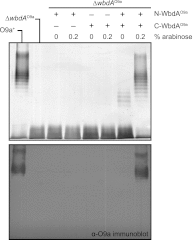
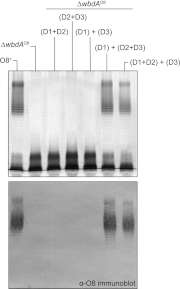
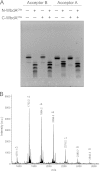
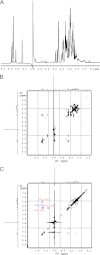
The Escherichia coli O9a and O8 polymannose O-polysaccharides (O-PSs) serve as model systems for the biosynthesis of bacterial polysaccharides by ATP-binding cassette transporter-dependent pathways. Both O-PSs contain a conserved primer-adaptor domain at the reducing terminus and a serotype-specific repeat unit domain. The repeat unit domain is polymerized by the serotype-specific WbdA mannosyltransferase. In serotype O9a, WbdA is a bifunctional α-(1→2)-, α-(1→3)-mannosyltransferase, and its counterpart in serotype O8 is trifunctional (α-(1→2), α-(1→3), and β-(1→2)). Little is known about the detailed structures or mechanisms of action of the WbdA polymerases, and here we establish that they are multidomain enzymes. WbdA(O9a) contains two separable and functionally active domains, whereas WbdA(O8) possesses three. In WbdC(O9a) and WbdB(O9a), substitution of the first Glu of the EX(7)E motif had detrimental effects on the enzyme activity, whereas substitution of the second had no significant effect on activity in vivo. Mutation of the Glu residues in the EX(7)E motif of the N-terminal WbdA(O9a) domain resulted in WbdA variants unable to synthesize O-PS. In contrast, mutation of the Glu residues in the motif of the C-terminal WbdA(O9a) domain generated an enzyme capable of synthesizing an altered O-PS repeat unit consisting of only α-(1→2) linkages. In vitro assays with synthetic acceptors unequivocally confirmed that the N-terminal domain of WbdA(O9a) possesses α-(1→2)-mannosyltransferase activity. Together, these studies form a framework for detailed structure-function studies on individual domains and a strategy applicable for dissection and analysis of other multidomain glycosyltransferases.
Figures




References
-
- Stenutz R., Weintraub A., Widmalm G. (2006) The structures of Escherichia coli O-polysaccharide antigens. FEMS Microbiol. Rev. 30, 382–403 - PubMed
-
- Sperandeo P., Dehò G., Polissi A. (2009) The lipopolysaccharide transport system of Gram-negative bacteria. Biochim. Biophys. Acta 1791, 594–602 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

