Activation of cannabinoid receptor type 1 (Cb1r) disrupts hepatic insulin receptor signaling via cyclic AMP-response element-binding protein H (Crebh)-mediated induction of Lipin1 gene
- PMID: 22989885
- PMCID: PMC3488074
- DOI: 10.1074/jbc.M112.377978
Activation of cannabinoid receptor type 1 (Cb1r) disrupts hepatic insulin receptor signaling via cyclic AMP-response element-binding protein H (Crebh)-mediated induction of Lipin1 gene
Abstract
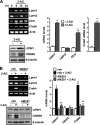
Activation of hepatic cannabinoid 1 receptor (Cb1r) signaling has been implicated in the development of phenotypes associated with fatty liver, hypertriglyceridemia, and insulin resistance. In the current study, we have elucidated the critical role of endoplasmic reticulum-bound transcription factor cyclic AMP-response element-binding protein H (Crebh) in mediating activated Cb1r signaling in inducing phosphatidic acid phosphatase Lipin1 gene expression and subsequently deregulating hepatic insulin receptor signaling. Cb1r agonist (2-arachidonoylglycerol (2-AG)) treatment induced Lipin1 gene expression in a Crebh-dependent manner via recruiting CREBH to the endogenous Lipin1 gene promoter. Adenoviral overexpression of Crebh or 2-AG treatment in mice induced Lipin1 gene expression to increase the hepatic diacylglycerol (DAG) level and phosphorylation of protein kinase Cε (PKCε). This in turn inhibited hepatic insulin receptor signaling. Knockdown of Crebh or Cb1r antagonism attenuated 2-AG-mediated induction of Lipin1 gene expression and decreased DAG production in mouse liver and subsequently restored insulin receptor signaling. Similarly, knockdown of Lipin1 attenuated the 2-AG-induced increase in the DAG level and PKCε phosphorylation. Finally, shRNA-mediated knockdown of Crebh partially but significantly blunted Lipin1 expression and the DAG level in db/db mice. These results demonstrate a novel mechanism by which Cb1r signaling induces Lipin1 gene expression and increases DAG production by activating Crebh, thereby deregulating insulin receptor signaling pathway and lipid homeostasis.
Figures



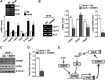
References
-
- Nogueiras R., Veyrat-Durebex C., Suchanek P. M., Klein M., Tschöp J., Caldwell C., Woods S. C., Wittmann G., Watanabe M., Liposits Z., Fekete C., Reizes O., Rohner-Jeanrenaud F., Tschöp M. H. (2008) Peripheral, but not central, CB1 antagonism provides food intake-independent metabolic benefits in diet-induced obese rats. Diabetes 57, 2977–2991 - PMC - PubMed
-
- Monteleone P., Matias I., Martiadis V., De Petrocellis L., Maj M., Di Marzo V. (2005) Blood levels of the endocannabinoid anandamide are increased in anorexia nervosa and in binge-eating disorder, but not in bulimia nervosa. Neuropsychopharmacology 30, 1216–1221 - PubMed
-
- Matias I., Gonthier M. P., Orlando P., Martiadis V., De Petrocellis L., Cervino C., Petrosino S., Hoareau L., Festy F., Pasquali R., Roche R., Maj M., Pagotto U., Monteleone P., Di Marzo V. (2006) Regulation, function, and dysregulation of endocannabinoids in models of adipose and β-pancreatic cells and in obesity and hyperglycemia. J. Clin. Endocrinol. Metab. 91, 3171–3180 - PubMed
-
- Jeong W. I., Osei-Hyiaman D., Park O., Liu J., Bátkai S., Mukhopadhyay P., Horiguchi N., Harvey-White J., Marsicano G., Lutz B., Gao B., Kunos G. (2008) Paracrine activation of hepatic CB1 receptors by stellate cell-derived endocannabinoids mediates alcoholic fatty liver. Cell Metab. 7, 227–235 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

