Estrous cycle variations in GABA(A) receptor phosphorylation enable rapid modulation by anabolic androgenic steroids in the medial preoptic area
- PMID: 22989919
- PMCID: PMC3490028
- DOI: 10.1016/j.neuroscience.2012.09.014
Estrous cycle variations in GABA(A) receptor phosphorylation enable rapid modulation by anabolic androgenic steroids in the medial preoptic area
Abstract
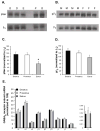
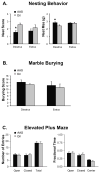
Anabolic androgenic steroids (AAS), synthetic testosterone derivatives that are used for ergogenic purposes, alter neurotransmission and behaviors mediated by GABA(A) receptors. Some of these effects may reflect direct and rapid action of these synthetic steroids at the receptor. The ability of other natural allosteric steroid modulators to alter GABA(A) receptor-mediated currents is dependent upon the phosphorylation state of the receptor complex. Here we show that phosphorylation of the GABA(A) receptor complex immunoprecipitated by β(2)/β(3) subunit-specific antibodies from the medial preoptic area (mPOA) of the mouse varies across the estrous cycle; with levels being significantly lower in estrus. Acute exposure to the AAS, 17α-methyltestosterone (17α-MeT), had no effect on the amplitude or kinetics of inhibitory postsynaptic currents in the mPOA of estrous mice when phosphorylation was low, but increased the amplitude of these currents from mice in diestrus, when it was high. Inclusion of the protein kinase C (PKC) inhibitor, calphostin, in the recording pipette eliminated the ability of 17α-MeT to enhance currents from diestrous animals, suggesting that PKC-receptor phosphorylation is critical for the allosteric modulation elicited by AAS during this phase. In addition, a single injection of 17α-MeT was found to impair an mPOA-mediated behavior (nest building) in diestrus, but not in estrus. PKC is known to target specific serine residues in the β(3) subunit of the GABA(A) receptor. Although phosphorylation of these β(3) serine residues showed a similar profile across the cycle, as did phosphoserine in mPOA lysates immunoprecipitated with β2/β3 antibody (lower in estrus than in diestrus or proestrus), the differences were not significant. These data suggest that the phosphorylation state of the receptor complex regulates both the ability of AAS to modulate receptor function in the mPOA and the expression of a simple mPOA-dependent behavior through a PKC-dependent mechanism that involves the β(3) subunit and other sites within the GABA(A) receptor complex.
Copyright © 2012 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures

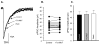
Similar articles
-
Anabolic steroids induce region- and subunit-specific rapid modulation of GABA(A) receptor-mediated currents in the rat forebrain.J Neurophysiol. 2000 Jun;83(6):3299-309. doi: 10.1152/jn.2000.83.6.3299. J Neurophysiol. 2000. Retraction in: J Neurophysiol. 2007 Sep;98(3):1841. doi: 10.1152/jn.z9k-8459-Retraction.2007. PMID: 10848550 Retracted.
-
Anabolic androgenic steroids induce age-, sex-, and dose-dependent changes in GABA(A) receptor subunit mRNAs in the mouse forebrain.Neuropharmacology. 2002 Sep;43(4):634-45. doi: 10.1016/s0028-3908(02)00154-5. Neuropharmacology. 2002. PMID: 12367608
-
The function and the expression of forebrain GABA(A) receptors change with hormonal state in the adult mouse.J Neurobiol. 2002 Feb 5;50(2):137-49. doi: 10.1002/neu.10021. J Neurobiol. 2002. PMID: 11793360
-
Anabolic androgenic steroids and forebrain GABAergic transmission.Neuroscience. 2006;138(3):793-9. doi: 10.1016/j.neuroscience.2005.08.039. Epub 2005 Nov 28. Neuroscience. 2006. PMID: 16310317 Review.
-
Anabolic androgenic steroid abuse: multiple mechanisms of regulation of GABAergic synapses in neuroendocrine control regions of the rodent forebrain.J Neuroendocrinol. 2012 Jan;24(1):202-14. doi: 10.1111/j.1365-2826.2011.02151.x. J Neuroendocrinol. 2012. PMID: 21554430 Free PMC article. Review.
Cited by
-
Plasticity of paternity: Effects of fatherhood on synaptic, intrinsic and morphological characteristics of neurons in the medial preoptic area of male California mice.Behav Brain Res. 2019 Jun 3;365:89-102. doi: 10.1016/j.bbr.2019.02.029. Epub 2019 Feb 22. Behav Brain Res. 2019. PMID: 30802534 Free PMC article.
-
Sex and exercise interact to alter the expression of anabolic androgenic steroid-induced anxiety-like behaviors in the mouse.Horm Behav. 2014 Jul;66(2):283-97. doi: 10.1016/j.yhbeh.2014.04.008. Epub 2014 Apr 21. Horm Behav. 2014. PMID: 24768711 Free PMC article.
-
Mad men, women and steroid cocktails: a review of the impact of sex and other factors on anabolic androgenic steroids effects on affective behaviors.Psychopharmacology (Berl). 2016 Feb;233(4):549-69. doi: 10.1007/s00213-015-4193-6. Epub 2016 Jan 12. Psychopharmacology (Berl). 2016. PMID: 26758282 Free PMC article. Review.
-
The role of AMP-activated protein kinase in the androgenic potentiation of cannabinoid-induced changes in energy homeostasis.Am J Physiol Endocrinol Metab. 2015 Mar 15;308(6):E482-95. doi: 10.1152/ajpendo.00421.2014. Epub 2014 Dec 30. Am J Physiol Endocrinol Metab. 2015. PMID: 25550281 Free PMC article.
-
Tyrosine phosphorylation of GABAA receptor γ2-subunit regulates tonic and phasic inhibition in the thalamus.J Neurosci. 2013 Jul 31;33(31):12718-27. doi: 10.1523/JNEUROSCI.0388-13.2013. J Neurosci. 2013. PMID: 23904608 Free PMC article.
References
-
- Ansonoff MA, Etgen AM. Estradiol elevates protein kinase C catalytic activity in the preoptic area of female rats. Endocrinology. 1998;139(7):3050–3056. - PubMed
-
- Basaria S. Androgen abuse in athletes: Detection and consequences. J Clin Encodrinol Metab. 2010;95:1533–1543. - PubMed
-
- Bitran D, Hilvers RJ, Kellogg CK. Ovarian endocrine status modulates the anxiolytic potency of diazepam and the efficacy of γ-aminobutyric acid-benzodiazepine receptor-mediated chloride ion transport. Behav Neurosci. 1991;105(5):653–662. - PubMed
-
- Blasberg ME, Langan CJ, Clark AS. The effects of 17α-methyltestosterone, methandrostenolone, and nandrolone decanoate on the rat estrous cycle. Physiol Behav. 1997;61(2):265–272. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

