Hypothalamic AgRP-neurons control peripheral substrate utilization and nutrient partitioning
- PMID: 22990237
- PMCID: PMC3501217
- DOI: 10.1038/emboj.2012.250
Hypothalamic AgRP-neurons control peripheral substrate utilization and nutrient partitioning
Abstract
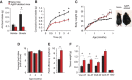
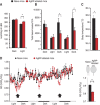
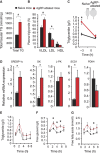
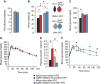
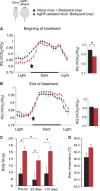
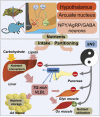
Obesity-related diseases such as diabetes and dyslipidemia result from metabolic alterations including the defective conversion, storage and utilization of nutrients, but the central mechanisms that regulate this process of nutrient partitioning remain elusive. As positive regulators of feeding behaviour, agouti-related protein (AgRP) producing neurons are indispensible for the hypothalamic integration of energy balance. Here, we demonstrate a role for AgRP-neurons in the control of nutrient partitioning. We report that ablation of AgRP-neurons leads to a change in autonomic output onto liver, muscle and pancreas affecting the relative balance between lipids and carbohydrates metabolism. As a consequence, mice lacking AgRP-neurons become obese and hyperinsulinemic on regular chow but display reduced body weight gain and paradoxical improvement in glucose tolerance on high-fat diet. These results provide a direct demonstration of a role for AgRP-neurons in the coordination of efferent organ activity and nutrient partitioning, providing a mechanistic link between obesity and obesity-related disorders.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

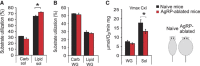
Comment in
-
AgRP neurons: a switch between peripheral carbohydrate and lipid utilization.EMBO J. 2012 Nov 14;31(22):4252-4. doi: 10.1038/emboj.2012.287. Epub 2012 Oct 19. EMBO J. 2012. PMID: 23085989 Free PMC article.
References
-
- Ahren B (2000) Autonomic regulation of islet hormone secretion—implications for health and disease. Diabetologia 43: 393–410 - PubMed
-
- Ahren B, Taborsky G, Porte D (1986) Neuropeptidergic versus cholinergic and adrenergic regulation of islet hormone secretion. Diabetologia 29: 827–836 - PubMed
-
- Bewick GA, Gardiner JV, Dhillo WS, Kent AS, White NE, Webster Z, Ghatei MA, Bloom SR (2005) Post-embryonic ablation of AgRP neurons in mice leads to a lean, hypophagic phenotype. FASEB J 19: 1680–1682 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

