Endocytic tubules regulated by Rab GTPases 5 and 11 are used for envelopment of herpes simplex virus
- PMID: 22990238
- PMCID: PMC3492727
- DOI: 10.1038/emboj.2012.262
Endocytic tubules regulated by Rab GTPases 5 and 11 are used for envelopment of herpes simplex virus
Abstract
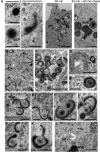
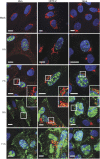
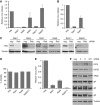
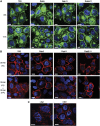
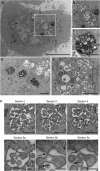
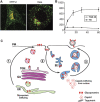
Enveloped viruses employ diverse and complex strategies for wrapping at cellular membranes, many of which are poorly understood. Here, an ultrastructural study of herpes simplex virus 1 (HSV1)-infected cells revealed envelopment in tubular membranes. These tubules were labelled by the fluid phase marker horseradish peroxidase (HRP), and were observed to wrap capsids as early as 2 min after HRP addition, indicating that the envelope had recently cycled from the cell surface. Consistent with this, capsids did not colocalise with either the trans-Golgi network marker TGN46 or late endosomal markers, but showed coincidence with the transferrin receptor. Virus glycoproteins were retrieved from the plasma membrane (PM) to label wrapping capsids, a process that was dependent on both dynamin and Rab5. Combined depletion of Rab5 and Rab11 reduced virus yield to <1%, resulting in aberrant localisation of capsids. These results suggest that endocytosis from the PM into endocytic tubules provides the main source of membrane for HSV1, and reveal a new mechanism for virus exploitation of the endocytic pathway.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Comment in
-
Viral infection: gift wrapped by the plasma membrane.Nat Rev Microbiol. 2012 Nov;10(11):732. doi: 10.1038/nrmicro2905. Nat Rev Microbiol. 2012. PMID: 23070554 No abstract available.
Similar articles
-
Novel Role for ESCRT-III Component CHMP4C in the Integrity of the Endocytic Network Utilized for Herpes Simplex Virus Envelopment.mBio. 2021 May 11;12(3):e02183-20. doi: 10.1128/mBio.02183-20. mBio. 2021. PMID: 33975940 Free PMC article.
-
Herpes Simplex Virus Entry by a Nonconventional Endocytic Pathway.J Virol. 2020 Nov 23;94(24):e01910-20. doi: 10.1128/JVI.01910-20. Print 2020 Nov 23. J Virol. 2020. PMID: 33028710 Free PMC article.
-
Rab6 dependent post-Golgi trafficking of HSV1 envelope proteins to sites of virus envelopment.Traffic. 2014 Feb;15(2):157-78. doi: 10.1111/tra.12134. Epub 2013 Nov 18. Traffic. 2014. PMID: 24152084 Free PMC article.
-
Role of Rab GTPases in HSV-1 infection: Molecular understanding of viral maturation and egress.Microb Pathog. 2018 May;118:146-153. doi: 10.1016/j.micpath.2018.03.028. Epub 2018 Mar 16. Microb Pathog. 2018. PMID: 29551438 Review.
-
Herpesvirus Nuclear Egress across the Outer Nuclear Membrane.Viruses. 2021 Nov 24;13(12):2356. doi: 10.3390/v13122356. Viruses. 2021. PMID: 34960625 Free PMC article. Review.
Cited by
-
The dynamic nature of the conserved tegument protein UL37 of herpesviruses.J Biol Chem. 2018 Oct 12;293(41):15827-15839. doi: 10.1074/jbc.RA118.004481. Epub 2018 Aug 30. J Biol Chem. 2018. PMID: 30166339 Free PMC article.
-
Exocytosis of Progeny Infectious Varicella-Zoster Virus Particles via a Mannose-6-Phosphate Receptor Pathway without Xenophagy following Secondary Envelopment.J Virol. 2020 Jul 30;94(16):e00800-20. doi: 10.1128/JVI.00800-20. Print 2020 Jul 30. J Virol. 2020. PMID: 32493818 Free PMC article.
-
Crystal structure of the herpesvirus inner tegument protein UL37 supports its essential role in control of viral trafficking.J Virol. 2014 May;88(10):5462-73. doi: 10.1128/JVI.00163-14. Epub 2014 Mar 5. J Virol. 2014. PMID: 24599989 Free PMC article.
-
Potential Effects of Hyperglycemia on SARS-CoV-2 Entry Mechanisms in Pancreatic Beta Cells.Viruses. 2024 Aug 2;16(8):1243. doi: 10.3390/v16081243. Viruses. 2024. PMID: 39205219 Free PMC article. Review.
-
In situ structure of virus capsids within cell nuclei by correlative light and cryo-electron tomography.Sci Rep. 2020 Oct 19;10(1):17596. doi: 10.1038/s41598-020-74104-x. Sci Rep. 2020. PMID: 33077791 Free PMC article.
References
-
- Bard F, Malhotra V (2006) The formation of TGN-to-plasma-membrane transport carriers. Annu Rev Cell Dev Biol 22: 439–455 - PubMed
-
- Barman S, Ali A, Hui EK, Adhikary L, Nayak DP (2001) Transport of viral proteins to the apical membranes and interaction of matrix protein with glycoproteins in the assembly of influenza viruses. Virus Res 77: 61–69 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

