Progress and Trends in Complement Therapeutics
- PMID: 22990692
- PMCID: PMC3526805
- DOI: 10.1007/978-1-4614-4118-2_1
Progress and Trends in Complement Therapeutics
Abstract
The past few years have proven to be a highly successful and exciting period for the field of complement-directed drug discovery and development. Driven by promising experiences with the first marketed complement drugs, increased knowledge about the involvement of complement in health and disease, and improvements in structural and analytical techniques as well as animal models of disease, the field has seen a surge in creative approaches to therapeutically intervene at various stages of the cascade. An impressive panel of compounds that show promise in clinical trials is meanwhile being lined up in the pipelines of both small biotechnology and big pharmaceutical companies. Yet with this new focus on complement-targeted therapeutics, important questions concerning target selection, point and length of intervention, safety, and drug delivery emerge. In view of the diversity of the clinical disorders involving abnormal complement activity or regulation, which include both acute and chronic diseases and affect a wide range of organs, diverse yet specifically tailored therapeutic approaches may be needed to shift complement back into balance. This chapter highlights the key changes in the field that shape our current perception of complement-targeted drugs and provides a brief overview of recent strategies and emerging trends. Selected examples of complement-related diseases and inhibitor classes are highlighted to illustrate the diversity and creativity in field.
Figures
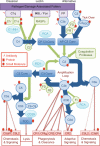

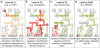
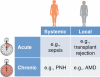


References
-
- Ahmad M, Pyaram K, Mullick J, Sahu A. Viral complement regulators: the expert mimicking swindlers. Indian J Biochem Biophys. 2007;44(5):331–343. - PubMed
-
- Alexion . Soliris® (eculizumab) Approved by FDA for All Patients with Atypical Hemolytic Uremic Syndrome (aHUS) Alexion Pharmaceuticals Press Release; 2011.
-
- Alfinito F, Ruggiero G, Sica M, Udhayachandran A, Rubino V, Pepa RD, et al. Eculizumab treatment modifies the immune profile of PNH patients. Immunobiology. 2011;217(7):698–703. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

