Ca(V)1.2 I-II linker structure and Timothy syndrome
- PMID: 22990809
- PMCID: PMC3536733
- DOI: 10.4161/chan.22078
Ca(V)1.2 I-II linker structure and Timothy syndrome
Abstract
Ca(V) channels are multi-subunit protein complexes that enable inward cellular Ca(2+) currents in response to membrane depolarization. We recently described structure-function studies of the intracellular α1 subunit domain I-II linker, directly downstream of domain IS6. The results show the extent of the linker's helical structure to be subfamily dependent, as dictated by highly conserved primary sequence differences. Moreover, the difference in structure confers different biophysical properties, particularly the extent and kinetics of voltage and calcium-dependent inactivation. Timothy syndrome is a human genetic disorder due to mutations in the Ca(V)1.2 gene. Here, we explored whether perturbation of the I-II linker helical structure might provide a mechanistic explanation for a Timothy syndrome mutant's (human Ca(V)1.2 G406R equivalent) biophysical effects on inactivation and activation. The results are equivocal, suggesting that a full mechanistic explanation for this Timothy syndrome mutation requires further investigation.
Figures
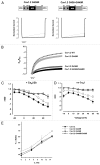
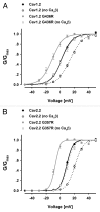
Erratum for
- Almagor L, Chomsky-Hecht O, Ben-Mocha A, Hendin-Barak D, Dascal N, Hirsch JA. The role of a voltage-dependent Ca2+ channel intracellular linker: a structure-function analysis. J Neurosci. 2012;32:7602–13. doi: 10.1523/JNEUROSCI.5727-11.2012 .
Similar articles
-
Restoration of normal L-type Ca2+ channel function during Timothy syndrome by ablation of an anchoring protein.Circ Res. 2011 Jul 22;109(3):255-61. doi: 10.1161/CIRCRESAHA.111.248252. Epub 2011 Jun 23. Circ Res. 2011. PMID: 21700933 Free PMC article.
-
Timothy mutation disrupts the link between activation and inactivation in Ca(V)1.2 protein.J Biol Chem. 2011 Sep 9;286(36):31557-64. doi: 10.1074/jbc.M111.255273. Epub 2011 Jun 17. J Biol Chem. 2011. PMID: 21685391 Free PMC article.
-
Elevated basal transcription can underlie timothy channel association with autism related disorders.Prog Neurobiol. 2020 Aug;191:101820. doi: 10.1016/j.pneurobio.2020.101820. Epub 2020 May 11. Prog Neurobiol. 2020. PMID: 32437834
-
Timothy syndrome iPSC modeling.Mol Cell Neurosci. 2020 Sep;107:103529. doi: 10.1016/j.mcn.2020.103529. Epub 2020 Jul 3. Mol Cell Neurosci. 2020. PMID: 32629111 Review.
-
Calcium Channel Mutations in Cardiac Arrhythmia Syndromes.Curr Mol Pharmacol. 2015;8(2):133-42. doi: 10.2174/1874467208666150518114857. Curr Mol Pharmacol. 2015. PMID: 25981977 Free PMC article. Review.
Cited by
-
Structural flexibility of CaV1.2 and CaV2.2 I-II proximal linker fragments in solution.Biophys J. 2013 Jun 4;104(11):2392-400. doi: 10.1016/j.bpj.2013.04.034. Biophys J. 2013. PMID: 23746511 Free PMC article.
-
Identification and Functional Characterization of a Novel CACNA1C-Mediated Cardiac Disorder Characterized by Prolonged QT Intervals With Hypertrophic Cardiomyopathy, Congenital Heart Defects, and Sudden Cardiac Death.Circ Arrhythm Electrophysiol. 2015 Oct;8(5):1122-32. doi: 10.1161/CIRCEP.115.002745. Epub 2015 Aug 7. Circ Arrhythm Electrophysiol. 2015. PMID: 26253506 Free PMC article.
-
Dysfunctional Cav1.2 channel in Timothy syndrome, from cell to bedside.Exp Biol Med (Maywood). 2019 Sep;244(12):960-971. doi: 10.1177/1535370219863149. Epub 2019 Jul 19. Exp Biol Med (Maywood). 2019. PMID: 31324123 Free PMC article.
References
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Miscellaneous
