Expression profiles of the nuclear receptors and their transcriptional coregulators during differentiation of neural stem cells
- PMID: 22990992
- PMCID: PMC3781591
- DOI: 10.1055/s-0032-1321789
Expression profiles of the nuclear receptors and their transcriptional coregulators during differentiation of neural stem cells
Abstract
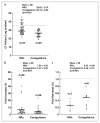
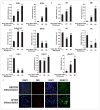
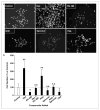
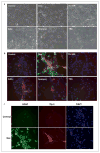
Neural stem cells (NSCs) are pluripotent precursors with the ability to proliferate and differentiate into 3 neural cell lineages, neurons, astrocytes and oligodendrocytes. Elucidation of the mechanisms underlying these biologic processes is essential for understanding both physiologic and pathologic neural development and regeneration after injury. Nuclear hormone receptors (NRs) and their transcriptional coregulators also play crucial roles in neural development, functions and fate. To identify key NRs and their transcriptional regulators in NSC differentiation, we examined mRNA expression of 49 NRs and many of their coregulators during differentiation (0-5 days) of mouse embryonic NSCs induced by withdrawal of fibroblast growth factor-2 (FGF2). 37 out of 49 NRs were expressed in NSCs before induction of differentiation, while receptors known to play major roles in neural development, such as THRα, RXRs, RORs, TRs, and COUP-TFs, were highly expressed. CAR, which plays important roles in xenobiotic metabolism, was also highly expressed. FGF2 withdrawal induced mRNA expression of RORγ, RXRγ, and MR by over 20-fold. Most of the transcriptional coregulators examined were expressed basally and throughout differentiation without major changes, while FGF2 withdrawal strongly induced mRNA expression of several histone deacetylases (HDACs), including HDAC11. Dexamethasone and aldosterone, respectively a synthetic glucocorticoid and natural mineralocorticoid, increased NSC numbers and induced differentiation into neurons and astrocytes. These results indicate that the NRs and their coregulators are present and/or change their expression during NSC differentiation, suggesting that they may influence development of the central nervous system in the absence or presence of their ligands.
© Georg Thieme Verlag KG Stuttgart · New York.
Conflict of interest statement
The authors do not have any conflict of interest.
Figures

References
-
- Burris TP. The nuclear receptor superfamily. In: Burris TP, McCabe ERB, editors. Nuclear receptors and genetic disease. London: Academic Press; 2001. pp. 1–58.
-
- Horn S, Heuer H. Thyroid hormone action during brain development: More questions than answers. Mol Cell Endocrinol. 2010;315:19–26. - PubMed
-
- Wolkowitz OM, Burke H, Epel ES, Reus VI. Glucocorticoids. Mood, memory, and mechanisms. Ann NY Acad Sci. 2009;1179:19–40. - PubMed
-
- Hughes ZA, Liu F, Marquis K, Muniz L, Pangalos MN, Ring RH, Whiteside GT, Brandon NJ. Estrogen receptor neurobiology and its potential for translation into broad spectrum therapeutics for CNS disorders. Curr Mol Pharmacol. 2009;2:215–236. - PubMed
-
- Kino T, Chrousos GP. Glucocorticoid effect on gene expression. In: Steckler T, Kalin NH, Reul JMHM, editors. Handbook on Stress and the Brain. Amsterdam: Elsevier BV; 2005. pp. 295–312.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

