The γ-carbonic anhydrase subcomplex of mitochondrial complex I is essential for development and important for photomorphogenesis of Arabidopsis
- PMID: 22991283
- PMCID: PMC3490601
- DOI: 10.1104/pp.112.204339
The γ-carbonic anhydrase subcomplex of mitochondrial complex I is essential for development and important for photomorphogenesis of Arabidopsis
Abstract
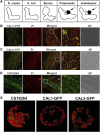
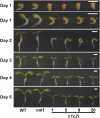
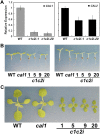
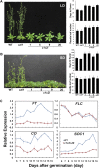
Complex I (NADH:ubiquinone oxidoreductase) is the entry point for electrons into the respiratory electron transport chain; therefore, it plays a central role in cellular energy metabolism. Complex I from different organisms has a similar basic structure. However, an extra structural module, referred to as the γ-carbonic anhydrase (γCA) subcomplex, is found in the mitochondrial complex I of photoautotrophic eukaryotes, such as green alga and plants, but not in that of the heterotrophic eukaryotes, such as fungi and mammals. It has been proposed that the γCA subcomplex is required for the light-dependent life style of photoautotrophic eukaryotes, but this hypothesis has not been successfully tested. We report here a genetic study of the genes γCAL1 and γCAL2 that encode two subunits of the γCA subcomplex of mitochondrial complex I. We found that mutations of γCAL1 and γCAL2 in Arabidopsis (Arabidopsis thaliana) result in defective embryogenesis and nongerminating seeds, demonstrating the functional significance of the γCA subcomplex of mitochondrial complex I in plant development. Surprisingly, we also found that reduced expression of γCAL1 and γCAL2 genes altered photomorphogenic development. The γcal1 mutant plant expressing the RNA interference construct of the γCAL2 gene showed a partial constitutive photomorphogenic phenotype in young seedlings and a reduced photoperiodic sensitivity in adult plants. The involvement of the γCA subcomplex of mitochondrial complex I in plant photomorphogenesis and the possible evolutionary significance of this plant-specific mitochondrial protein complex are discussed.
Figures



References
-
- Bauer D, Viczián A, Kircher S, Nobis T, Nitschke R, Kunkel T, Panigrahi KC, Adám E, Fejes E, Schäfer E, et al. (2004) Constitutive photomorphogenesis 1 and multiple photoreceptors control degradation of phytochrome interacting factor 3, a transcription factor required for light signaling in Arabidopsis. Plant Cell 16: 1433–1445 - PMC - PubMed
-
- Bäurle I, Dean C. (2006) The timing of developmental transitions in plants. Cell 125: 655–664 - PubMed
-
- Brauna H-P, Zabaleta E. (2007) Carbonic anhydrase subunits of the mitochondrial NADH dehydrogenase complex (complex I) in plants. Physiol Plant 129: 114–122
-
- Bultema JB, Braun HP, Boekema EJ, Kouril R. (2009) Megacomplex organization of the oxidative phosphorylation system by structural analysis of respiratory supercomplexes from potato. Biochim Biophys Acta 1787: 60–67 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

