The integration and functional evaluation of rabbit pacing cells transplanted into the left ventricular free wall
- PMID: 22991489
- PMCID: PMC3444971
- DOI: 10.7150/ijms.4971
The integration and functional evaluation of rabbit pacing cells transplanted into the left ventricular free wall
Abstract
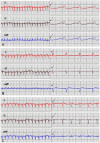
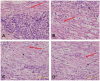
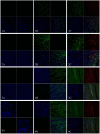
To evaluate the feasibility of cell transplantation to treat bradyarrhythmia, we analyzed the in vivo integration and pacing function after transplantation of mHCN4-modified rabbit bone marrow mesenchymal stem cells (MSCs) into the rabbit left ventricle free wall epicardium. In our investigation, we injected MSCs transduced with or without mHCN4 into the rabbit left ventricle free wall epicardium. Chemical ablation of the sinoatrial node was performed and bilateral vagus nerves were sequentially stimulated to observe premature left ventricular contraction or left ventricular rhythm. We found that the mHCN4-transduced MSC group had a significantly higher ventricular rate and a shorter QRS duration than that of the control and EGFP group. Furthermore, the mHCN4-transduced MSCs, but not the control cells, gradually adapted long-spindle morphology and became indistinguishable from adjacent ventricle myocytes. The modified MSCs showed pacing function approximately 1 week after transplantation and persisted at least 4 weeks after transplantation. In conclusion, a bradyarrhythmia model can be successfully established by chemical ablation of the sinoatrial node and sequential bilateral vagus nerve stimulation. The mHCN4-modified rabbit MSCs displayed evident dynamic morphology changes after being transplanted into rabbit left ventricle free wall epicardium. Our studies may provide a promising strategy of using modified stem cell transplantation to treat bradyarrhythmia.
Keywords: biological pacing.; bone marrow mesenchymal stem cell; mHCN4; subepicardial transplantation.
Conflict of interest statement
Conflict of Interests: The authors have declared that no conflict of interest exists.
Figures


Similar articles
-
In situ investigation of allografted mouse HCN4 gene-transfected rat bone marrow mesenchymal stromal cells with the use of patch-clamp recording of ventricular slices.Cytotherapy. 2013 Aug;15(8):905-19. doi: 10.1016/j.jcyt.2013.03.010. Epub 2013 Jun 13. Cytotherapy. 2013. PMID: 23768927
-
mHCN4 genetically modified canine mesenchymal stem cells provide biological pacemaking function in complete dogs with atrioventricular block.Pacing Clin Electrophysiol. 2013 Sep;36(9):1138-49. doi: 10.1111/pace.12154. Epub 2013 May 10. Pacing Clin Electrophysiol. 2013. PMID: 23663261
-
[Experimental study on xenogenic sino-atrial nodal tissue transplanted into left ventricular wall].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2010 Feb;24(2):226-9. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2010. PMID: 20187459 Chinese.
-
Canine bone marrow mesenchymal stromal cells with lentiviral mHCN4 gene transfer create cardiac pacemakers.Cytotherapy. 2012 May;14(5):529-39. doi: 10.3109/14653249.2012.654490. Epub 2012 Feb 8. Cytotherapy. 2012. PMID: 22316056
-
Impact of physiologic pacing versus right ventricular pacing among patients with left ventricular ejection fraction greater than 35%: A systematic review for the 2018 ACC/AHA/HRS guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society.Heart Rhythm. 2019 Sep;16(9):e280-e298. doi: 10.1016/j.hrthm.2018.10.035. Epub 2018 Nov 6. Heart Rhythm. 2019. PMID: 30412776
Cited by
-
Pacemaker Channels and the Chronotropic Response in Health and Disease.Circ Res. 2024 May 10;134(10):1348-1378. doi: 10.1161/CIRCRESAHA.123.323250. Epub 2024 May 9. Circ Res. 2024. PMID: 38723033 Free PMC article. Review.
-
Enhancement of pacing function by HCN4 overexpression in human pluripotent stem cell-derived cardiomyocytes.Stem Cell Res Ther. 2022 Apr 1;13(1):141. doi: 10.1186/s13287-022-02818-y. Stem Cell Res Ther. 2022. PMID: 35365232 Free PMC article.
-
Bioengineering the Cardiac Conduction System: Advances in Cellular, Gene, and Tissue Engineering for Heart Rhythm Regeneration.Front Bioeng Biotechnol. 2021 Aug 2;9:673477. doi: 10.3389/fbioe.2021.673477. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34409019 Free PMC article. Review.
References
-
- Robinson RB, Siegelbaum SA. Hyperpolarization-activated cation currents: from molecules to physiological function. Annu Rev Physiol. 2003;65:453–80. - PubMed
-
- Zagotta WN, Olivier NB, Black KD, Young EC, Olson R, Gouaux E. Structural basis for modulation and agonist specificity of HCN pacemaker channels. Nature. 2003;425:200–5. - PubMed
-
- Verkerk AO, van Borren MM, Peters RJ, Broekhuis E, Lam KY, Coronel R. et al. Single cells isolated from human sinoatrial node: action potentials and numerical reconstruction of pacemaker current. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:904–7. - PubMed
-
- Verkerk AO, Wilders R, van Borren MM, Peters RJ, Broekhuis E, Lam K. et al. Pacemaker current (I(f)) in the human sinoatrial node. Eur Heart J. 2007;28:2472–8. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

