Interferon-gamma-induced nitric oxide inhibits the proliferation of murine renal cell carcinoma cells
- PMID: 22991499
- PMCID: PMC3445049
- DOI: 10.7150/ijbs.4694
Interferon-gamma-induced nitric oxide inhibits the proliferation of murine renal cell carcinoma cells
Abstract
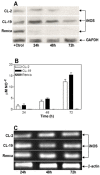
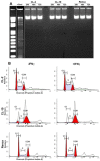
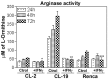
Renal cell carcinoma (RCC) remains one of the most resistant tumors to systemic chemotherapy, radiotherapy, and immunotherapy. Despite great progress in understanding the basic biology of RCC, the rate of responses in animal models and clinical trials using interferons (IFNs) has not improved significantly. It is likely that the lack of responses can be due to the tumor's ability to develop tumor escape strategies. Currently, the use of targeted therapies has improved the clinical outcomes of patients with RCC and is associated with an increase of Th1-cytokine responses (IFNγ), indicating the importance of IFNγ in inhibiting tumor proliferation. Thus, the present study was designed to investigate a new mechanism by which IFNγ mediates direct anti-proliferative effects against murine renal cell carcinoma cell lines. When cultured RCC cell lines were exposed to murine recombinant IFNγ, a dose dependent growth inhibition in CL-2 and CL-19 cells was observed; this effect was not observed in Renca cells. Growth inhibition in CL-2 and CL-19 cell lines was associated with the intracellular induction of nitric oxide synthase (iNOS) protein, resulting in a sustained elevation of nitric oxide (NO) and citrulline, and a decrease in arginase activity. The inhibition of cell proliferation appears to be due to an arrest in the cell cycle. The results indicate that in certain RCC cell lines, IFNγ modulates L-arginine metabolism by shifting from arginase to iNOS activity, thereby developing a potent inhibitory mechanism to encumber tumor cell proliferation and survival. Elucidating the cellular events triggered by IFNγ in murine RCC cell lines will permit anti-tumor effects to be exploited in the development of new combination therapies that interfere with L-arginine metabolism to effectively combat RCC in patients.
Keywords: Interferon-gamma; L-arginine; arginase 2; cell proliferation.; nitric oxide; nitric oxide synthase; polyamines; renal cell carcinoma.
Conflict of interest statement
Conflict of Interest: All authors have declared that no conflicts of interest exist and agree with the contents of the manuscript for publication.
Figures



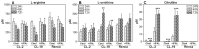

References
-
- Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011 Jul;61(4):212–36. - PubMed
-
- Staehler M, Rohrmann K, Bachmann A, Zaak D, Stief CG, Siebels M. Therapeutic approaches in metastatic renal cell carcinoma. BJU Int. 2005 Jun;95(8):1153–61. - PubMed
-
- Bleumer I, Oosterwijk E, De MP, Mulders PF. Immunotherapy for renal cell carcinoma. Eur Urol. 2003 Jul;44(1):65–75. - PubMed
-
- Atkins MB, Regan M, McDermott D. Update on the role of interleukin 2 and other cytokines in the treatment of patients with stage IV renal carcinoma. Clin Cancer Res. 2004 Sep 15;10(18 Pt 2):6342S–6S. - PubMed
-
- McDermott DF, Regan MM, Clark JI, Flaherty LE, Weiss GR, Logan TF, Kirkwood JM, Gordon MS, Sosman JA, Ernstoff MS, Tretter CP, Urba WJ. et al. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol. 2005 Jan 1;23(1):133–41. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

