Development and validation of a cisplatin dose-ototoxicity model
- PMID: 22992258
- PMCID: PMC5549622
- DOI: 10.3766/jaaa.23.7.3
Development and validation of a cisplatin dose-ototoxicity model
Abstract
Background: Cisplatin is effective in the treatment of several cancers but is a known ototoxin resulting in shifts to hearing sensitivity in up to 50-60% of patients. Cisplatin-induced hearing shifts tend to occur first within an octave of a patient's high frequency hearing limit, termed the sensitive range for ototoxicity (SRO), and progress to lower frequencies. While it is currently not possible to know which patients will experience ototoxicity without testing their hearing directly, monitoring the SRO provides an early indication of damage. A tool to help forecast susceptibility to ototoxic-induced changes in the SRO in advance of each chemotherapy treatment visit may prove useful for ototoxicity monitoring efforts, patient counseling, and therapeutic planning.
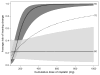
Purpose: This project was designed to (1) establish pretreatment risk curves that quantify the probability that a new patient will suffer hearing loss within the SRO during treatment with cisplatin and (2) evaluate the accuracy of these predictions in an independent sample of Veterans receiving cisplatin for the treatment of cancer.
Study sample: Two study samples were used. The Developmental sample contained 23 subjects while the Validation sample consisted of 12 subjects.
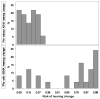
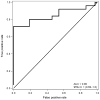
Data collection and analysis: Risk curve predictions for SRO threshold shifts following cisplatin exposure were developed using a Developmental sample comprised of data from a total of 155 treatment visits obtained in 45 ears of 23 Veterans. Pure-tone thresholds were obtained within each subject's SRO at each treatment visit and compared with baseline measures. The risk of incurring an SRO shift was statistically modeled as a function of factors related to chemotherapy treatment (cisplatin dose, radiation treatment, doublet medication) and patient status (age, pre-exposure hearing, cancer location and stage). The model was reduced so that only statistically significant variables were included. Receiver-operating characteristic (ROC) curve analyses were then used to determine the accuracy of the risk curve predictions in an independent Validation sample of observations from over 62 treatment visits obtained in 24 ears of 12 Veterans.
Results: Only cumulative cisplatin dose and pre-exposure hearing were found to be significantly related to the risk for hearing shift. The dose-ototoxicity risk curve predictions developed from the Developmental sample yielded area under the ROC curve accuracy estimates of 0.85 when applied to an independent Validation sample.
Conclusions: Cumulative cisplatin dose in combination with pre-exposure hearing provides an indication of whether hearing will shift in the SRO in advance of cisplatin administration. The validated dose-ototoxicity risk curves described herein can be used before and during treatment to anticipate hearing loss. While having such a tool would not replace serial hearing testing, it would be of great benefit to an ototoxicity monitoring program. It would promote relevant pretreatment counseling. Furthermore, for those found to be at risk of SRO shifts within the speech frequencies, the oncology treatment plan could incorporate anticipated dosing adjustments that could stave off the impact that ototoxicity might bring.
American Academy of Audiology.
Figures
Similar articles
-
ABR obtained from time-efficient train stimuli for cisplatin ototoxicity monitoring.J Am Acad Audiol. 2013 Oct;24(9):769-81. doi: 10.3766/jaaa.24.9.2. J Am Acad Audiol. 2013. PMID: 24224985 Free PMC article.
-
Evaluation of audiometric threshold shift criteria for ototoxicity monitoring.J Am Acad Audiol. 2010 May;21(5):301-14; quiz 357. doi: 10.3766/jaaa.21.5.3. J Am Acad Audiol. 2010. PMID: 20569665 Free PMC article.
-
Factors affecting sensitivity of distortion-product otoacoustic emissions to ototoxic hearing loss.Ear Hear. 2008 Dec;29(6):875-93. doi: 10.1097/AUD.0b013e318181ad99. Ear Hear. 2008. PMID: 18753950 Clinical Trial.
-
Ototoxicity monitoring in children treated with platinum chemotherapy.Int J Audiol. 2018 Sep;57(sup4):S34-S40. doi: 10.1080/14992027.2017.1355570. Epub 2017 Jul 24. Int J Audiol. 2018. PMID: 28737048 Review.
-
Applying U.S. national guidelines for ototoxicity monitoring in adult patients: perspectives on patient populations, service gaps, barriers and solutions.Int J Audiol. 2018 Sep;57(sup4):S3-S18. doi: 10.1080/14992027.2017.1398421. Epub 2017 Nov 20. Int J Audiol. 2018. PMID: 29157038 Free PMC article. Review.
Cited by
-
Mechanisms of Aminoglycoside- and Cisplatin-Induced Ototoxicity.Am J Audiol. 2021 Oct 11;30(3S):887-900. doi: 10.1044/2021_AJA-21-00006. Epub 2021 Aug 20. Am J Audiol. 2021. PMID: 34415784 Free PMC article. Review.
-
A Store-and-Forward Tele-Audiology Solution to Promote Efficient Screenings for Ototoxicity during Cisplatin Cancer Treatment.J Am Acad Audiol. 2015 Oct;26(9):750-60. doi: 10.3766/jaaa.15028. J Am Acad Audiol. 2015. PMID: 26415968 Free PMC article.
-
Gene transfection mediated by polyethyleneimine-polyethylene glycol nanocarrier prevents cisplatin-induced spiral ganglion cell damage.Neural Regen Res. 2015 Mar;10(3):425-31. doi: 10.4103/1673-5374.153691. Neural Regen Res. 2015. PMID: 25878591 Free PMC article.
-
Accuracy of distortion-product otoacoustic emissions-based ototoxicity monitoring using various primary frequency step-sizes.Int J Audiol. 2012 Sep;51(9):689-96. doi: 10.3109/14992027.2012.688143. Epub 2012 Jun 7. Int J Audiol. 2012. PMID: 22676700 Free PMC article.
-
Prospective longitudinal assessment of sensorineural hearing loss with hyperfractionated radiation therapy alone in patients with average-risk medulloblastoma.Neurooncol Pract. 2014 Sep;1(3):86-93. doi: 10.1093/nop/npu017. Epub 2014 Aug 2. Neurooncol Pract. 2014. PMID: 31386031 Free PMC article.
References
-
- American Academy of Audiology (Academy) American Academy of Audiology Position Statement and Clinical Practice Guidelines: Ototoxicity Monitoring. 2009 http://www.audiology.org/resources/documentlibrary/Documents/OtoMonGuide....
-
- Edge S, Byrd D, Compton C, Fritz A, Greene F, Trotti A III, editors. American Joint Committee on Cancer (AJCC) Cancer Staging Manual. New York: Springer; 2010.
-
- American Speech-Language-Hearing Association Ad Hoc Committee on Audiologic Management of Individuals Receiving Ototoxic and/or Vestibulotoxic Drug Therapy. Audiologic Management of Individuals Receiving Cochleotoxic Drug Therapy. 1994 http://www.asha.org/docs/html/GL1994-00003.html.
-
- Arora R, Thakur JS, Azad RK, Mohindroo NK, Sharma DR, Seam RK. Cisplatin-based chemotherapy: add high-frequency audiometry in the regimen. Indian J Cancer. 2009;46(4):311–317. - PubMed
-
- Bertolini P, Lassalle M, Mercier G, et al. Platinum compound-related ototoxicity in children: long-term follow-up reveals continuous worsening of hearing loss. J Pediatr Hematol Oncol. 2004;26(10):649–655. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
