Antenatal corticosteroids trial in preterm births to increase neonatal survival in developing countries: study protocol
- PMID: 22992312
- PMCID: PMC3477119
- DOI: 10.1186/1742-4755-9-22
Antenatal corticosteroids trial in preterm births to increase neonatal survival in developing countries: study protocol
Abstract
Background: Preterm birth is a major cause of neonatal mortality, responsible for 28% of neonatal deaths overall. The administration of antenatal corticosteroids to women at high risk of preterm birth is a powerful perinatal intervention to reduce neonatal mortality in resource rich environments. The effect of antenatal steroids to reduce mortality and morbidity among preterm infants in hospital settings in developed countries with high utilization is well established, yet they are not routinely used in developing countries. The impact of increasing antenatal steroid use in hospital or community settings with low utilization rates and high infant mortality among premature infants due to lack of specialized services has not been well researched. There is currently no clear evidence about the safety of antenatal corticosteroid use for community-level births.
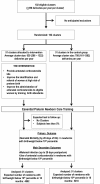
Methods: We hypothesize that a multi country, two-arm, parallel cluster randomized controlled trial to evaluate whether a multifaceted intervention to increase the use of antenatal corticosteroids, including components to improve the identification of pregnancies at high risk of preterm birth and providing and facilitating the appropriate use of steroids, will reduce neonatal mortality at 28 days of life in preterm newborns, compared with the standard delivery of care in selected populations of six countries. 102 clusters in Argentina, Guatemala, Kenya, India, Pakistan, and Zambia will be randomized, and around 60,000 women and newborns will be enrolled. Kits containing vials of dexamethasone, syringes, gloves, and instructions for administration will be distributed. Improving the identification of women at high risk of preterm birth will be done by (1) diffusing recommendations for antenatal corticosteroids use to health providers, (2) training health providers on identification of women at high risk of preterm birth, (3) providing reminders to health providers on the use of the kits, and (4) using a color-coded tape to measure uterine height to estimate gestational age in women with unknown gestational age. In both intervention and control clusters, health providers will be trained in essential newborn care for low birth weight babies. The primary outcome is neonatal mortality at 28 days of life in preterm infants.
Trial registration: ClinicalTrials.gov NCT01084096.
Figures



References
-
- United Nations Millennium Declaration. United Nations General Assembly Resolution 55/2. United Nations, New York, NY; 2000. Available at: http://www.un.org/millennium/declaration/ares552e.pdf. Accessed December 28, 2005.
-
- National Institutes of Health. consensus development conference statement. The Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. 1994. Available at: 2005. http://consensus.nih.gov/1994/1994AntenatalSteroidPerinatal095html.htm. Accessed December 28, 2005.
-
- Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database of Systematic Reviews. 2006. DOI: 10.1002/14651858.CD004454.pub2. Art. No.: CD004454. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

