Voltage-gated sodium channels and metastatic disease
- PMID: 22992466
- PMCID: PMC3508774
- DOI: 10.4161/chan.21910
Voltage-gated sodium channels and metastatic disease
Abstract
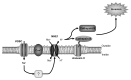
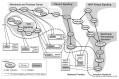
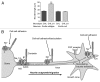
Voltage-gated Na (+) channels (VGSCs) are macromolecular protein complexes containing a pore-forming α subunit and smaller non-pore-forming β subunits. VGSCs are expressed in metastatic cells from a number of cancers. In these cells, Na (+) current carried by α subunits enhances migration, invasion and metastasis in vivo. In contrast, the β subunits mediate cellular adhesion and process extension. The prevailing hypothesis is that VGSCs are upregulated in cancer, in general favoring an invasive/metastatic phenotype, although the mechanisms are still not fully clear. Expression of the Nav 1.5 α subunit associates with poor prognosis in clinical breast cancer specimens, suggesting that VGSCs may have utility as prognostic markers for cancer progression. Furthermore, repurposing existing VGSC-blocking therapeutic drugs may provide a new strategy to improve outcomes in patients suffering from metastatic disease, which is the major cause of cancer-related deaths, and for which there is currently no cure.
Figures

References
-
- Catterall WA. Cellular and molecular biology of voltage-gated sodium channels. Physiol Rev. 1992;72:S15–48. - PubMed
-
- Hille B. Ionic channels of excitable membranes. Sunderland (Massachusetts): Sinauer Associates Inc., 1992.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
