In vivo multiphoton imaging of mitochondrial structure and function during acute kidney injury
- PMID: 22992467
- PMCID: PMC4136483
- DOI: 10.1038/ki.2012.328
In vivo multiphoton imaging of mitochondrial structure and function during acute kidney injury
Abstract
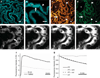
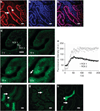
Mitochondrial dysfunction has been implicated in the pathogenesis of acute kidney injury due to ischemia and toxic drugs. Methods for imaging mitochondrial function in cells using confocal microscopy are well established; more recently, it was shown that these techniques can be utilized in ex vivo kidney tissue using multiphoton microscopy. We extended this approach in vivo and found that kidney mitochondrial structure and function can be imaged in anesthetized rodents using multiphoton excitation of endogenous and exogenous fluorophores. Mitochondrial nicotinamide adenine dinucleotide increased markedly in rat kidneys in response to ischemia. Following intravenous injection, the mitochondrial membrane potential-dependent dye TMRM was taken up by proximal tubules; in response to ischemia, the membrane potential dissipated rapidly and mitochondria became shortened and fragmented in proximal tubules. In contrast, the mitochondrial membrane potential and structure were better maintained in distal tubules. Changes in mitochondrial structure, nicotinamide adenine dinucleotide, and membrane potential were found in the proximal, but not distal, tubules after gentamicin exposure. These changes were sporadic, highly variable among animals, and were preceded by changes in non-mitochondrial structures. Thus, real-time changes in mitochondrial structure and function can be imaged in rodent kidneys in vivo using multiphoton excitation of endogenous and exogenous fluorophores in response to ischemia-reperfusion injury or drug toxicity.
Figures


References
-
- Duchen MR, Szabadkai G. Roles of mitochondria in human disease. Essays Biochem. 2010;47:115–137. - PubMed
-
- Plotnikov EY, Kazachenko AV, Vyssokikh MY, et al. The role of mitochondria in oxidative and nitrosative stress during ischemia/reperfusion in the rat kidney. Kidney Int. 2007;72:1493–1502. - PubMed
-
- Burke TJ, Wilson DR, Levi M, et al. Role of mitochondria in ischemic acute renal failure. Clin Exp Dial Apheresis. 1983;7:49–61. - PubMed
-
- Sun Z, Zhang X, Ito K, et al. Amelioration of oxidative mitochondrial DNA damage and deletion after renal ischemic injury by the KATP channel opener diazoxide. Am J Physiol Renal Physiol. 2008;294:F491–F498. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

