Pancortins interact with amyloid precursor protein and modulate cortical cell migration
- PMID: 22992957
- PMCID: PMC3472593
- DOI: 10.1242/dev.082909
Pancortins interact with amyloid precursor protein and modulate cortical cell migration
Abstract
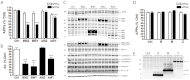
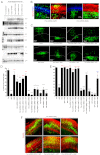
Neuronal precursor cell migration in the developing mammalian brain is a complex process requiring the coordinated interaction of numerous proteins. We have recently shown that amyloid precursor protein (APP) plays a role in migration into the cortical plate through its interaction with two cytosolic signaling proteins, disabled 1 (DAB1) and disrupted in schizophrenia 1 (DISC1). In order to identify extracellular factors that may signal through APP to regulate migration, we performed an unbiased mass spectrometry-based screen for factors that bind to the extracellular domain of APP in the rodent brain. Through this screen, we identified an interaction between APP and pancortins, proteins expressed throughout the developing and mature cerebral cortex. Via co-immunoprecipitation, we show that APP interacts with all four of the mammalian pancortin isoforms (AMY, AMZ, BMY, BMZ). We demonstrate that the BMZ and BMY isoforms of pancortin can specifically reduce β-secretase- but not α-secretase-mediated cleavage of endogenous APP in cell culture, suggesting a biochemical consequence of the association between pancortins and APP. Using in utero electroporation to overexpress and knock down specific pancortin isoforms, we reveal a novel role for pancortins in migration into the cortical plate. Interestingly, we observe opposing roles for alternate pancortin isoforms, with AMY overexpression and BMZ knock down both preventing proper migration of neuronal precursor cells. Finally, we show that BMZ can partially rescue a loss of APP expression and that APP can rescue effects of AMY overexpression, suggesting that pancortins act in conjunction with APP to regulate entry into the cortical plate. Taken together, these results suggest a biochemical and functional interaction between APP and pancortins, and reveal a previously unidentified role for pancortins in mammalian cortical development.
Figures





References
-
- Araki W., Kitaguchi N., Tokushima Y., Ishii K., Aratake H., Shimohama S., Nakamura S., Kimura J. (1991). Trophic effect of β-amyloid precursor protein on cerebral cortical neurons in culture. Biochem. Biophys. Res. Commun. 181, 265-271 - PubMed
-
- Bai Y., Markham K., Chen F., Weerasekera R., Watts J., Horne P., Wakutani Y., Bagshaw R., Mathews P. M., Fraser P. E., et al. (2008). The in vivo brain interactome of the amyloid precursor protein. Mol. Cell. Proteomics 7, 15-34 - PubMed
-
- Barembaum M., Moreno T. A., LaBonne C., Sechrist J., Bronner-Fraser M. (2000). Noelin-1 is a secreted glycoprotein involved in generation of the neural crest. Nat. Cell Biol. 2, 219-225 - PubMed
-
- Bulfone A., Smiga S. M., Shimamura K., Peterson A., Puelles L., Rubenstein J. L. (1995). T-brain-1: a homolog of Brachyury whose expression defines molecularly distinct domains within the cerebral cortex. Neuron 15, 63-78 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

