Amyloid plaque formation precedes dendritic spine loss
- PMID: 22993126
- PMCID: PMC3508278
- DOI: 10.1007/s00401-012-1047-8
Amyloid plaque formation precedes dendritic spine loss
Abstract
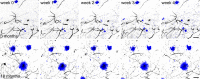
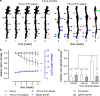
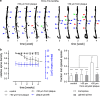
Amyloid-beta plaque deposition represents a major neuropathological hallmark of Alzheimer's disease. While numerous studies have described dendritic spine loss in proximity to plaques, much less is known about the kinetics of these processes. In particular, the question as to whether synapse loss precedes or follows plaque formation remains unanswered. To address this question, and to learn more about the underlying kinetics, we simultaneously imaged amyloid plaque deposition and dendritic spine loss by applying two-photon in vivo microscopy through a cranial window in double transgenic APPPS1 mice. As a result, we first observed that the rate of dendritic spine loss in proximity to plaques is the same in both young and aged animals. However, plaque size only increased significantly in the young cohort, indicating that spine loss persists even many months after initial plaque appearance. Tracking the fate of individual spines revealed that net spine loss is caused by increased spine elimination, with the rate of spine formation remaining constant. Imaging of dendritic spines before and during plaque formation demonstrated that spine loss around plaques commences at least 4 weeks after initial plaque formation. In conclusion, spine loss occurs, shortly but with a significant time delay, after the birth of new plaques, and persists in the vicinity of amyloid plaques over many months. These findings hence give further hope to the possibility that there is a therapeutic window between initial amyloid plaque deposition and the onset of structural damage at spines.
Figures
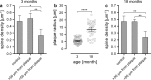
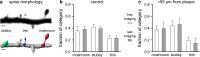

References
-
- Bittner T, Fuhrmann M, Burgold S, Jung CKE, Volbracht C, Steiner H, Mitteregger G, Kretzschmar HA, Haass C, Herms J. Gamma-secretase inhibition reduces spine density in vivo via an amyloid precursor protein-dependent pathway. J Neurosci Off J Soc Neurosci. 2009;29:10405–10409. doi: 10.1523/JNEUROSCI.2288-09.2009. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

