Ataxia telangiectasia mutated kinase controls chronic gammaherpesvirus infection
- PMID: 22993144
- PMCID: PMC3497635
- DOI: 10.1128/JVI.00917-12
Ataxia telangiectasia mutated kinase controls chronic gammaherpesvirus infection
Abstract
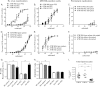
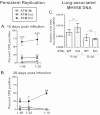
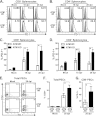
Gammaherpesviruses, such as Epstein-Barr virus (EBV), are ubiquitous cancer-associated pathogens that interact with DNA damage response, a tumor suppressor network. Chronic gammaherpesvirus infection and pathogenesis in a DNA damage response-insufficient host are poorly understood. Ataxia-telangiectasia (A-T) is associated with insufficiency of ataxia-telangiectasia mutated (ATM), a critical DNA damage response kinase. A-T patients display a pattern of anti-EBV antibodies suggestive of poorly controlled EBV replication; however, parameters of chronic EBV infection and pathogenesis in the A-T population remain unclear. Here we demonstrate that chronic gammaherpesvirus infection is poorly controlled in an animal model of A-T. Intriguingly, in spite of a global increase in T cell activation and numbers in wild-type (wt) and ATM-deficient mice in response to mouse gammaherpesvirus 68 (MHV68) infection, the generation of an MHV68-specific immune response was altered in the absence of ATM. Our finding that ATM expression is necessary for an optimal adaptive immune response against gammaherpesvirus unveils an important connection between DNA damage response and immune control of chronic gammaherpesvirus infection, a connection that is likely to impact viral pathogenesis in an ATM-insufficient host.
Figures




Comment in
-
Testing for herpesvirus infection is essential in children with chromosomal-instability syndromes.J Virol. 2013 Mar;87(6):3616-7. doi: 10.1128/JVI.03279-12. J Virol. 2013. PMID: 23436901 Free PMC article. No abstract available.
-
Reply to "testing for herpesvirus infection is essential in children with chromosomal-instability syndromes".J Virol. 2013 Mar;87(6):3618. doi: 10.1128/JVI.03401-12. J Virol. 2013. PMID: 23436902 Free PMC article. No abstract available.
Similar articles
-
Testing for herpesvirus infection is essential in children with chromosomal-instability syndromes.J Virol. 2013 Mar;87(6):3616-7. doi: 10.1128/JVI.03279-12. J Virol. 2013. PMID: 23436901 Free PMC article. No abstract available.
-
Reply to "testing for herpesvirus infection is essential in children with chromosomal-instability syndromes".J Virol. 2013 Mar;87(6):3618. doi: 10.1128/JVI.03401-12. J Virol. 2013. PMID: 23436902 Free PMC article. No abstract available.
-
B Cell-Specific Expression of Ataxia-Telangiectasia Mutated Protein Kinase Promotes Chronic Gammaherpesvirus Infection.J Virol. 2017 Sep 12;91(19):e01103-17. doi: 10.1128/JVI.01103-17. Print 2017 Oct 1. J Virol. 2017. PMID: 28701397 Free PMC article.
-
Epstein-Barr Virus (EBV)-Related Lymphoproliferative Disorders in Ataxia Telangiectasia: Does ATM Regulate EBV Life Cycle?Front Immunol. 2019 Jan 4;9:3060. doi: 10.3389/fimmu.2018.03060. eCollection 2018. Front Immunol. 2019. PMID: 30662441 Free PMC article. Review.
-
The ATM protein kinase and cellular redox signaling: beyond the DNA damage response.Trends Biochem Sci. 2012 Jan;37(1):15-22. doi: 10.1016/j.tibs.2011.10.002. Epub 2011 Nov 11. Trends Biochem Sci. 2012. PMID: 22079189 Free PMC article. Review.
Cited by
-
Combination of proviral and antiviral roles of B cell-intrinsic STAT1 expression defines parameters of chronic gammaherpesvirus infection.mBio. 2024 Nov 13;15(11):e0159824. doi: 10.1128/mbio.01598-24. Epub 2024 Oct 23. mBio. 2024. PMID: 39440973 Free PMC article.
-
Testing for herpesvirus infection is essential in children with chromosomal-instability syndromes.J Virol. 2013 Mar;87(6):3616-7. doi: 10.1128/JVI.03279-12. J Virol. 2013. PMID: 23436901 Free PMC article. No abstract available.
-
Fatal Lymphoproliferative Disease in Two Siblings Lacking Functional FAAP24.J Clin Immunol. 2016 Oct;36(7):684-92. doi: 10.1007/s10875-016-0317-y. Epub 2016 Jul 29. J Clin Immunol. 2016. PMID: 27473539
-
Murine Gammaherpesvirus 68 LANA and SOX Homologs Counteract ATM-Driven p53 Activity during Lytic Viral Replication.J Virol. 2015 Dec 16;90(5):2571-85. doi: 10.1128/JVI.02867-15. J Virol. 2015. PMID: 26676792 Free PMC article.
-
Mutations in components of antiviral or microbial defense as a basis for breast cancer.Funct Integr Genomics. 2013 Nov;13(4):411-24. doi: 10.1007/s10142-013-0336-1. Epub 2013 Sep 21. Funct Integr Genomics. 2013. PMID: 24057274 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

