Overexpression of the ubiquitin-specific protease 7 resulting from transfection or mutations in the ICP0 binding site accelerates rather than depresses herpes simplex virus 1 gene expression
- PMID: 22993145
- PMCID: PMC3497670
- DOI: 10.1128/JVI.01981-12
Overexpression of the ubiquitin-specific protease 7 resulting from transfection or mutations in the ICP0 binding site accelerates rather than depresses herpes simplex virus 1 gene expression
Abstract
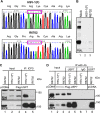
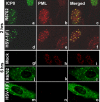
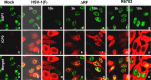
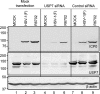
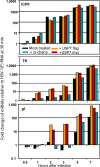
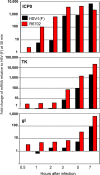
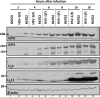
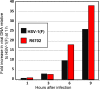
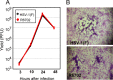
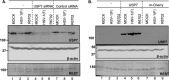
Earlier studies reported that ICP0, a key regulatory protein encoded by herpes simplex virus 1 (HSV-1), binds ubiquitin-specific protease 7 (USP7). The fundamental conclusion of these studies is that depletion of USP7 destabilized ICP0, that ICP0 mediated the degradation of USP7, and that amino acid substitutions in ICP0 that abolished binding to USP7 significantly impaired the ability of HSV-1 to replicate. We show here that, indeed, depletion of USP7 leads to reduction of ICP0 and that USP7 is degraded in an ICP0-dependent manner. However, overexpression of USP7 or substitution in ICP0 of a single amino acid to abolish binding to USP7 accelerated the accumulation of viral mRNAs and proteins at early times after infection and had no deleterious effect on virus yields. A clue as to why USP7 is degraded emerged from the observation that, notwithstanding the accelerated expression of viral genes, the plaques formed by the mutant virus were very small, implying a defect in virus transmission from cell to cell.
Figures
References
-
- Boutell C, Everett RD. 2003. The herpes simplex virus type 1 (HSV-1) regulatory protein ICP0 interacts with and ubiquitinates p53. J. Biol. Chem. 278:36596–36602 - PubMed
-
- Canning M, Boutell C, Parkinson J, Everett RD. 2004. A RING finger ubiquitin ligase is protected from autocatalyzed ubiquitination and degradation by binding to ubiquitin-specific protease USP7. J. Biol. Chem. 279:38160–38168 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

