Thymic function is maintained during Salmonella-induced atrophy and recovery
- PMID: 22993205
- PMCID: PMC3912538
- DOI: 10.4049/jimmunol.1200070
Thymic function is maintained during Salmonella-induced atrophy and recovery
Abstract
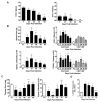
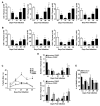
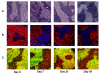
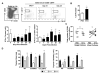
Thymic atrophy is a frequent consequence of infection with bacteria, viruses, and parasites and is considered a common virulence trait between pathogens. Multiple reasons have been proposed to explain this atrophy, including premature egress of immature thymocytes, increased apoptosis, or thymic shutdown to prevent tolerance to the pathogen from developing. The severe loss in thymic cell number can reflect an equally dramatic reduction in thymic output, potentially reducing peripheral T cell numbers. In this study, we examine the relationship between systemic Salmonella infection and thymic function. During infection, naive T cell numbers in peripheral lymphoid organs increase. Nevertheless, this occurs despite a pronounced thymic atrophy caused by viable bacteria, with a peak 50-fold reduction in thymocyte numbers. Thymic atrophy is not dependent upon homeostatic feedback from peripheral T cells or on regulation of endogenous glucocorticoids, as demonstrated by infection of genetically altered mice. Once bacterial numbers fall, thymocyte numbers recover, and this is associated with increases in the proportion and proliferation of early thymic progenitors. During atrophy, thymic T cell maturation is maintained, and single-joint TCR rearrangement excision circle analysis reveals there is only a modest fall in recent CD4(+) thymic emigrants in secondary lymphoid tissues. Thus, thymic atrophy does not necessarily result in a matching dysfunctional T cell output, and thymic homeostasis can constantly adjust to systemic infection to ensure that naive T cell output is maintained.
Figures


Similar articles
-
Differential susceptibility and maturation of thymocyte subsets during Salmonella Typhimurium infection: insights on the roles of glucocorticoids and Interferon-gamma.Sci Rep. 2017 Jan 16;7:40793. doi: 10.1038/srep40793. Sci Rep. 2017. PMID: 28091621 Free PMC article.
-
Comparative analysis of thymic subpopulations during different modes of atrophy identifies the reactive oxygen species scavenger, N-acetyl cysteine, to increase the survival of thymocytes during infection-induced and lipopolysaccharide-induced thymic atrophy.Immunology. 2019 May;157(1):21-36. doi: 10.1111/imm.13043. Epub 2019 Feb 11. Immunology. 2019. PMID: 30659606 Free PMC article.
-
Discontinued postnatal thymocyte development in sphingosine 1-phosphate-lyase-deficient mice.J Immunol. 2009 Oct 1;183(7):4292-301. doi: 10.4049/jimmunol.0901724. Epub 2009 Sep 11. J Immunol. 2009. PMID: 19748984
-
The thymus road to a T cell: migration, selection, and atrophy.Front Immunol. 2024 Aug 27;15:1443910. doi: 10.3389/fimmu.2024.1443910. eCollection 2024. Front Immunol. 2024. PMID: 39257583 Free PMC article. Review.
-
The thymus is a common target organ in infectious diseases.PLoS Pathog. 2006 Jun;2(6):e62. doi: 10.1371/journal.ppat.0020062. PLoS Pathog. 2006. PMID: 16846255 Free PMC article. Review.
Cited by
-
Accelerated Loss of TCR Repertoire Diversity in Common Variable Immunodeficiency.J Immunol. 2016 Sep 1;197(5):1642-9. doi: 10.4049/jimmunol.1600526. Epub 2016 Aug 1. J Immunol. 2016. PMID: 27481850 Free PMC article.
-
Immunity to intestinal pathogens: lessons learned from Salmonella.Immunol Rev. 2014 Jul;260(1):168-82. doi: 10.1111/imr.12184. Immunol Rev. 2014. PMID: 24942689 Free PMC article. Review.
-
Cholinergic epithelial cell with chemosensory traits in murine thymic medulla.Cell Tissue Res. 2014 Dec;358(3):737-48. doi: 10.1007/s00441-014-2002-x. Epub 2014 Oct 10. Cell Tissue Res. 2014. PMID: 25300645 Free PMC article.
-
Differential susceptibility and maturation of thymocyte subsets during Salmonella Typhimurium infection: insights on the roles of glucocorticoids and Interferon-gamma.Sci Rep. 2017 Jan 16;7:40793. doi: 10.1038/srep40793. Sci Rep. 2017. PMID: 28091621 Free PMC article.
-
Salmonella infection: Interplay between the bacteria and host immune system.Immunol Lett. 2017 Oct;190:42-50. doi: 10.1016/j.imlet.2017.07.006. Epub 2017 Jul 15. Immunol Lett. 2017. PMID: 28720334 Free PMC article. Review.
References
-
- Petrie HT, Zuniga-Pflucker JC. Zoned out: functional mapping of stromal signaling microenvironments in the thymus. Annu Rev Immunol. 2007;25:649–679. - PubMed
-
- Anderson G, Lane PJ, Jenkinson EJ. Generating intrathymic microenvironments to establish T-cell tolerance. Nat Rev Immunol. 2007;7:954–963. - PubMed
-
- Aspinall R, Mitchell W. Reversal of age-associated thymic atrophy: treatments, delivery, and side effects. Exp Gerontol. 2008;43:700–705. - PubMed
Publication types
MeSH terms
Grants and funding
- 090244/WT_/Wellcome Trust/United Kingdom
- BB/G023468/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- G0401620/MRC_/Medical Research Council/United Kingdom
- BB/F022778/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- G0900857/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Medical
Research Materials

