Comparison of immune responses to the O-specific polysaccharide and lipopolysaccharide of Vibrio cholerae O1 in Bangladeshi adult patients with cholera
- PMID: 22993410
- PMCID: PMC3491541
- DOI: 10.1128/CVI.00321-12
Comparison of immune responses to the O-specific polysaccharide and lipopolysaccharide of Vibrio cholerae O1 in Bangladeshi adult patients with cholera
Abstract
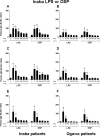
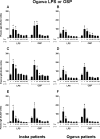
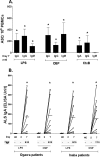
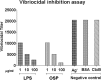
Immunity against Vibrio cholerae O1 is serogroup specific, and serogrouping is defined by the O-specific polysaccharide (OSP) part of lipopolysaccharide (LPS). Despite this, human immune responses to V. cholerae OSP have not previously been characterized. We assessed immune responses against V. cholerae OSP in adults with cholera caused by V. cholerae O1 El Tor serotype Inaba or Ogawa in Dhaka, Bangladesh, using O1 OSP-core-bovine serum albumin (OSPc:BSA) conjugates; responses targeted OSP in these conjugates. Responses of Inaba-infected patients to Inaba OSP and LPS increased significantly in IgG, IgM, and IgA isotypes from the acute to convalescent phases of illness, and the responses correlated well between OSP and LPS (R = 0.86, 0.73, and 0.91, respectively; P < 0.01). Plasma IgG, IgM, and IgA responses to Ogawa OSP and LPS in Ogawa-infected patients also correlated well with each other (R = 0.60, 0.60, and 0.92, respectively; P < 0.01). Plasma IgM responses to Inaba OSP and Ogawa OSP correlated with the respective serogroup-specific vibriocidal antibodies (R = 0.80 and 0.66, respectively; P < 0.001). Addition of either OSPc:BSA or LPS, but not BSA, to vibriocidal assays inhibited vibriocidal responses in a comparable and concentration-dependent manner. Mucosal IgA immune responses to OSP and LPS were also similar. Our study is the first to characterize anti-OSP immune responses in patients with cholera and suggests that responses targeting V. cholerae LPS, including vibriocidal responses that correlate with protection against cholera, predominantly target OSP. Induction of anti-OSP responses may be associated with protection against cholera, and our results may support the development of a vaccine targeting V. cholerae OSP.
Figures

Comment in
-
Insights from natural infection-derived immunity to cholera instruct vaccine efforts.Clin Vaccine Immunol. 2012 Nov;19(11):1707-11. doi: 10.1128/CVI.00543-12. Epub 2012 Sep 19. Clin Vaccine Immunol. 2012. PMID: 22993412 Free PMC article. No abstract available.
References
-
- Albert MJ, Alam K, Rahman AS, Huda S, Sack RB. 1994. Lack of cross-protection against diarrhea due to Vibrio cholerae O1 after oral immunization of rabbits with V. cholerae O139 Bengal. J. Infect. Dis. 169:709–710 - PubMed
-
- Cox AD, Brisson JR, Varma V, Perry MB. 1996. Structural analysis of the lipopolysaccharide from Vibrio cholerae O139. Carbohydr. Res. 290:43–58 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 AI077883/AI/NIAID NIH HHS/United States
- TW05572/TW/FIC NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- TW07144/TW/FIC NIH HHS/United States
- K01 TW007144/TW/FIC NIH HHS/United States
- R24 TW007988/TW/FIC NIH HHS/United States
- ImNIH/Intramural NIH HHS/United States
- K01 TW007409/TW/FIC NIH HHS/United States
- K01 TW07409/TW/FIC NIH HHS/United States
- K08 AI089721/AI/NIAID NIH HHS/United States
- TW005572/TW/FIC NIH HHS/United States
- K08 AI100923/AI/NIAID NIH HHS/United States
- U01 AI058935/AI/NIAID NIH HHS/United States
- D43 TW005572/TW/FIC NIH HHS/United States
- R03 AI063079/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

