Jedi-1 and MEGF10 signal engulfment of apoptotic neurons through the tyrosine kinase Syk
- PMID: 22993420
- PMCID: PMC3464495
- DOI: 10.1523/JNEUROSCI.6350-11.2012
Jedi-1 and MEGF10 signal engulfment of apoptotic neurons through the tyrosine kinase Syk
Abstract
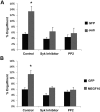
During the development of the peripheral nervous system there is extensive apoptosis, and these neuronal corpses need to be cleared to prevent an inflammatory response. Recently, Jedi-1 and MEGF10, both expressed in glial precursor cells, were identified in mouse as having an essential role in this phagocytosis (Wu et al., 2009); however, the mechanisms by which they promote engulfment remained unknown. Both Jedi-1 and MEGF10 are homologous to the Drosophila melanogaster receptor Draper, which mediates engulfment through activation of the tyrosine kinase Shark. Here, we identify Syk, the mammalian homolog of Shark, as a signal transducer for both Jedi-1 and MEGF10. Syk interacted with each receptor independently through the immunoreceptor tyrosine-based activation motifs (ITAMs) in their intracellular domains. The interaction was enhanced by phosphorylation of the tyrosines in the ITAMs by Src family kinases (SFKs). Jedi association with Syk and activation of the kinase was also induced by exposure to dead cells. Expression of either Jedi-1 or MEGF10 in HeLa cells facilitated engulfment of carboxylated microspheres to a similar extent, and there was no additive effect when they were coexpressed. Mutation of the ITAM tyrosines of Jedi-1 and MEGF10 prevented engulfment. The SFK inhibitor PP2 or a selective Syk inhibitor (BAY 61-3606) also blocked engulfment. Similarly, in cocultures of glial precursors and dying sensory neurons from embryonic mice, addition of PP2 or knock down of endogenous Syk decreased the phagocytosis of apoptotic neurons. These results indicate that both Jedi-1 and MEGF10 can mediate phagocytosis independently through the recruitment of Syk.
Figures







References
-
- Abram CL, Lowell CA. Convergence of immunoreceptor and integrin signaling. Immunol Rev. 2007;218:29–44. - PubMed
-
- Akakura S, Singh S, Spataro M, Akakura R, Kim JI, Albert ML, Birge RB. The opsonin MFG-E8 is a ligand for the alphavbeta5 integrin and triggers DOCK180-dependent Rac1 activation for the phagocytosis of apoptotic cells. Exp Cell Res. 2004;292:403–416. - PubMed
-
- Awasaki T, Tatsumi R, Takahashi K, Arai K, Nakanishi Y, Ueda R, Ito K. Essential role of the apoptotic cell engulfment genes draper and ced-6 in programmed axon pruning during Drosophila metamorphosis. Neuron. 2006;50:855–867. - PubMed
-
- Berton G, Mócsai A, Lowell CA. Src and Syk kinases: key regulators of phagocytic cell activation. Trends Immunol. 2005;26:208–214. - PubMed
-
- Bhavsar PJ, Vigorito E, Turner M, Ridley AJ. Vav GEFs regulate macrophage morphology and adhesion-induced Rac and Rho activation. Exp Cell Res. 2009;315:3345–3358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
