A role for silent synapses in the development of the pathway from layer 2/3 to 5 pyramidal cells in the neocortex
- PMID: 22993426
- PMCID: PMC6621489
- DOI: 10.1523/JNEUROSCI.1262-12.2012
A role for silent synapses in the development of the pathway from layer 2/3 to 5 pyramidal cells in the neocortex
Abstract
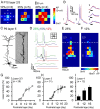
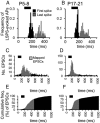
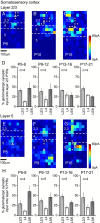
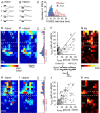
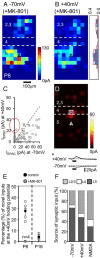
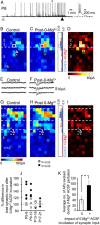
The integration of neurons within the developing cerebral cortex is a prolonged process dependent on a combination of molecular and physiological cues. To examine the latter we used laser scanning photostimulation (LSPS) of caged glutamate in conjunction with whole-cell patch-clamp electrophysiology to probe the integration of pyramidal cells in the sensorimotor regions of the mouse neocortex. In the days immediately after postnatal day 5 (P5) the origin of the LSPS-evoked AMPA receptor (AMPAR)-mediated synaptic inputs were diffuse and poorly defined with considerable variability between cells. Over the subsequent week this coalesced and shifted, primarily influenced by an increased contribution from layers 2/3 cells, which became a prominent motif of the afferent input onto layer 5 pyramidal cells regardless of cortical region. To further investigate this particular emergent translaminar connection, we alternated our mapping protocol between two holding potentials (-70 and +40 mV) allowing us to detect exclusively NMDA receptor (NMDAR)-mediated inputs. This revealed distal MK-801-sensitive synaptic inputs that predict the formation of the mature, canonical layer 2/3 to 5 pathway. However, these were a transient feature and had been almost entirely converted to AMPAR synapses at a later age (P16). To examine the role of activity in the recruitment of early NMDAR synapses, we evoked brief periods (20 min) of rhythmic bursting. Short intense periods of activity could cause a prolonged augmentation of the total input onto pyramidal cells up until P12; a time point when the canonical circuit has been instated and synaptic integration shifts to a more consolidatory phase.
Figures



References
-
- Abrahamsson T, Gustafsson B, Hanse E. AMPA silencing is a prerequisite for developmental long-term potentiation in the hippocampal CA1 region. J Neurophysiol. 2008;100:2605–2614. - PubMed
-
- Agmon A, Connors BW. Repetitive burst-firing neurons in the deep layers of mouse somatosensory cortex. Neurosci Lett. 1989;99:137–141. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
