Astrocyte-derived thrombospondins mediate the development of hippocampal presynaptic plasticity in vitro
- PMID: 22993427
- PMCID: PMC3475988
- DOI: 10.1523/JNEUROSCI.2604-12.2012
Astrocyte-derived thrombospondins mediate the development of hippocampal presynaptic plasticity in vitro
Abstract
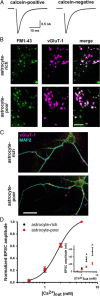
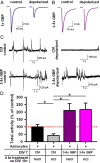
Astrocytes contribute to many neuronal functions, including synaptogenesis, but their role in the development of synaptic plasticity remains unclear. Presynaptic muting of hippocampal glutamatergic terminals defends against excitotoxicity. Here we studied the role of astrocytes in the development of presynaptic muting at glutamatergic synapses in rat hippocampal neurons. We found that astrocytes were critical for the development of depolarization-dependent and G(i/o)-dependent presynaptic muting. The ability of cAMP analogues to modulate presynaptic function was also impaired by astrocyte deficiency. Although astrocyte deprivation resulted in postsynaptic glutamate receptor deficits, this effect appeared independent of astrocytes' role in presynaptic muting. Muting was restored with chronic, but not acute, treatment with astrocyte-conditioned medium, indicating that a soluble factor is permissive for muting. Astrocyte-derived thrombospondins (TSPs) are likely responsible because TSP1 mimicked the effect of conditioned medium, and gabapentin, a high-affinity antagonist of TSP binding to the α2δ-1 calcium channel subunit, mimicked astrocyte deprivation. We found evidence that protein kinase A activity is abnormal in astrocyte-deprived neurons but restored by TSP1, so protein kinase A dysfunction may provide a mechanism by which muting is disrupted during astrocyte deficiency. In summary our results suggest an important role for astrocyte-derived TSPs, acting through α2δ-1, in maturation of a potentially important form of presynaptic plasticity.
Figures




References
-
- Adams JC, Tucker RP. The thrombospondin type 1 repeat (TSR) superfamily: diverse proteins with related roles in neuronal development. Dev Dyn. 2000;218:280–299. - PubMed
-
- Alexanian AR. Neural stem cells induce bone-marrow-derived mesenchymal stem cells to generate neural stem-like cells via juxtacrine and paracrine interactions. Exp Cell Res. 2005;310:383–391. - PubMed
-
- Amur-Umarjee S, Phan T, Campagnoni AT. Myelin basic protein mRNA translocation in oligodendrocytes is inhibited by astrocytes in vitro. J Neurosci Res. 1993;36:99–110. - PubMed
-
- Arikkath J, Campbell KP. Auxiliary subunits: essential components of the voltage-gated calcium channel complex. Curr Opin Neurobiol. 2003;13:298–307. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
