Peripheral ammonia as a mediator of methamphetamine neurotoxicity
- PMID: 22993432
- PMCID: PMC3464918
- DOI: 10.1523/JNEUROSCI.2530-12.2012
Peripheral ammonia as a mediator of methamphetamine neurotoxicity
Abstract
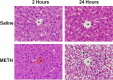
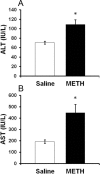
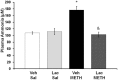
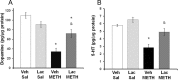
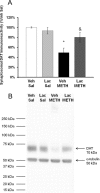
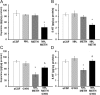
Ammonia is metabolized by the liver and has established neurological effects. The current study examined the possibility that ammonia contributes to the neurotoxic effects of methamphetamine (METH). The results show that a binge dosing regimen of METH to the rat increased plasma and brain ammonia concentrations that were paralleled by evidence of hepatotoxicity. The role of peripheral ammonia in the neurotoxic effects of METH was further substantiated by the demonstration that the enhancement of peripheral ammonia excretion blocked the increases in brain and plasma ammonia and attenuated the long-term depletions of dopamine and serotonin typically produced by METH. Conversely, the localized perfusion of ammonia in combination with METH, but not METH alone or ammonia alone, into the striatum recapitulated the neuronal damage produced by the systemic administration of METH. Furthermore, this damage produced by the local administration of ammonia and METH was blocked by the GYKI 52466 [4-(8-methyl-9H-1,3-dioxolo[4,5-h][2,3]benzodiazepin-5-yl)-benzamine hydrochloride], an AMPA receptor antagonist. These findings highlight the importance of ammonia derived from the periphery as a small-molecule mediator of METH neurotoxicity and more broadly emphasize the importance of peripheral organ damage as a possible mechanism that mediates the neuropathology produced by drugs of abuse and other neuroactive molecules.
Figures


References
-
- Ago M, Ago K, Hara K, Kashimura S, Ogata M. Toxicological and histopathological analysis of a patient who died nine days after a single intravenous dose of methamphetamine: a case report. Leg Med (Tokyo) 2006;8:235–239. - PubMed
-
- Batshaw ML, Hyman SL, Mellits ED, Thomas GH, DeMuro R, Coyle JT. Behavioral and neurotransmitter changes in the urease-infused rat: a model of congenital hyperammonemia. Pediatr Res. 1986;20:1310–1315. - PubMed
-
- Brown JM, Riddle EL, Sandoval V, Weston RK, Hanson JE, Crosby MJ, Ugarte YV, Gibb JW, Hanson GR, Fleckenstein AE. A single methamphetamine administration rapidly decreases vesicular dopamine uptake. J Pharmacol Exp Ther. 2002;302:497–501. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
