Short-term results of intravitreal dexamethasone implant (OZURDEX(®)) in treatment of recalcitrant diabetic macular edema: A case series
- PMID: 22993460
- PMCID: PMC3441033
- DOI: 10.4103/0974-620X.99368
Short-term results of intravitreal dexamethasone implant (OZURDEX(®)) in treatment of recalcitrant diabetic macular edema: A case series
Abstract
Context: Dexamethasone Posterior-Segment Drug Delivery System is a novel, biodegradable, sustained-release drug delivery system (OZURDEX(®)) for treatment of macular edema following retinal vein occlusion and posterior uveitis. However, its potential role in management of diabetic macular edema has not been reported yet.
Aim: The aim was to evaluate the safety and efficacy of (OZURDEX(®)) in patients with recalcitrant diabetic macular edema (DME).
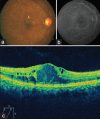
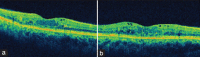
Setting and design: A retrospective, interventional case series from a tertiary eye care center in India is presented. Inclusion criteria comprised patients presenting with recalcitrant DME, 3 or more months after one or more treatments of macular laser photocoagulation and/or intravitreal anti-vascular endothelial growth factor (VEGF) injections. Exclusion criteria included history of corticosteroid-responsive intraocular pressure (IOP) rise, cataract extraction, or other intraocular surgery within 3 months. The main outcome measure was visual acuity at 1 and 4 months after OZURDEX(®) injection. Secondary outcome measures included change in central macular thickness on Optical coherence tomography (OCT) and changes in IOP following intravitreal OZURDEX(®) implant. Of 18 eyes (17 patients) with recalcitrant diabetic macular edema that underwent OZURDEX(®) implant, three eyes (two patients) had follow-up of more than 3 months post-injection.
Results: Mean age of patients was 56 years. Mean duration of diabetes mellitus was 16.6 years. Systemic control of DM was good as assessed by FBS/PPBS and HbA1c. The pre-operative mean central macular thickness was 744.3 μm and improved to 144 and 570 μm at months 1 and 4, respectively. Preoperative mean BCVA was 0.6 logMAR units and improved to 0.3 and 0.46 logMAR units at month 1 and 4, respectively. The mean follow-up was 4.3 months (range 4-5 months).
Conclusion: OZURDEX(®) appears efficacious in management of recalcitrant diabetic macular edema. The results of the ongoing POSURDEX(®) study will elaborate these effects better.
Keywords: Dexamethasone; OZURDEX®; diabetes mellitus; diabetic macular edema; intravitreal implant.
Conflict of interest statement
Figures
References
-
- Pelzek C, Lim JI. Diabetic macular edema: Review and update. Ophthalmol Clin North Am. 2002;15:555–63. - PubMed
-
- Funatsu H, Yamashita H, Noma H, Mimura T, Yamashita T, Hori S. Increased levels of vascular endothelial growth factor and interleukin-6 in the aqueous humor of diabetics with macular edema. Am J Ophthalmol. 2002;133:70–7. - PubMed
-
- Antonetti DA, Barber AJ, Khin S, Lieth E, Tarbell JM, Gardner TW Penn state retina research group. Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content: Vascular endothelial growth factor decreases occludin in retinal endothelial cells. Diabetes. 1998;47:1953–9. - PubMed
-
- Leopold IH. Nonsteroidal and steroidal anti-inflammatory agents. In: Sears M, Tarkkanen A, editors. Surgical pharmacology of the eye. New York NY: Raven Press; 1985. pp. 83–133.
-
- Nauck M, Karakiulakis G, Perruchoud A, Papakonstantinou E, Roth M. Corticosteroids inhibit the expression of the vascular endothelial growth factor gene in human vascular smooth muscle cells. Eur J Pharmacol. 1998;341:309–15. - PubMed
LinkOut - more resources
Full Text Sources

