Gemcitabine and paclitaxel suppress the production of vascular endothelial growth factor induced by deferoxamine in human non-small cell lung cancer A549 cells
- PMID: 22993609
- PMCID: PMC3445941
- DOI: 10.3892/etm.2010.130
Gemcitabine and paclitaxel suppress the production of vascular endothelial growth factor induced by deferoxamine in human non-small cell lung cancer A549 cells
Abstract
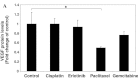
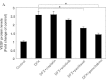
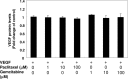
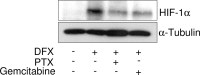
Vascular endothelial growth factor (VEGF) plays an important role in the process of angiogenesis in many types of cancer, including non-small cell lung cancer (NSCLC), and angiogenesis inhibitors and standard chemotherapy exhibit synergy though an unknown mechanism. We therefore hypothesized that cytotoxic chemotherapy influences VEGF production and analyzed VEGF production in an NSCLC A549 cell line after treatment with standard chemotherapy. Paclitaxel inhibited the production of VEGF in A549 cells, while cisplatin and erlotinib did not. Paclitaxel and gemcitabine inhibited deferoxamine (DFX) (known to mimic hypoxia)-induced VEGF production in A549 cells. Erlotinib also inhibited DFX-induced VEGF production in A549 cells slightly, while cisplatin did not. We subsequently examined the effect of the interaction between paclitaxel or gemcitabine and VEGF protein. Paclitaxel and gemcitabine did not directly affect the binding of VEGF. Since VEGF is known as one of the HIF-1 target genes, we examined the effect of paclitaxel and gemcitabine on HIF-1α levels induced by DFX in A549 cells. Paclitaxel and gemcitabine inhibited DFX-induced HIF-1α in A549 cells. These findings may be useful for future treatment schedules, including anti-cancer agents in NSCLC.
Figures


References
-
- Crabb SJ, Patsios D, Sauerbrei E, Ellis PM, Arnold A, Goss G, Leighl NB, Shepherd FA, Powers J, Seymour L, Laurie SA. Tumor cavitation: impact on objective response evaluation in trials of angiogenesis inhibitors in non-small-cell lung cancer. J Clin Oncol. 2009;27:404–410. - PubMed
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. - PubMed
-
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. - PubMed
-
- Dudek AZ, Mahaseth H. Circulating angiogenic cytokines in patients with advanced non-small cell lung cancer: correlation with treatment response and survival. Cancer Invest. 2005;23:193–200. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
