Influence of elastin-derived peptides on metalloprotease production in endothelial cells
- PMID: 22993640
- PMCID: PMC3445960
- DOI: 10.3892/etm.2010.157
Influence of elastin-derived peptides on metalloprotease production in endothelial cells
Abstract
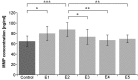
Matrix metalloproteases (MMPs) are a family of zinc-dependent endopeptidases that degrade extracellular matrix proteins. MMP-1 and MMP-2 are produced by endothelial cells and are involved in specific vascular pathologies, including atherosclerosis and aortal aneurysm. One of the most important differences between these two metalloproteases is the possibility of hydrolysis of elastin and collagen type IV by MMP-2, but not by MMP-1. Elastin-derived peptides are generated as a result of the degradation of elastin fibers. The aim of our study was to compare the production of MMP-1 and MMP-2 in cultured human arterial endothelial cells derived from vascular pathologies localized at three different sites, the coronary artery, iliac artery and aorta, measured as their concentration in cell culture medium. The second aim was to evaluate the influence of κ-elastin (at concentrations 0.1, 0.4, 1.0, 2.5 or 5.0 μg/ml) on the production of the evaluated metalloproteases in three endothelial cell lines. The production of MMP-1 was statistically significantly greater in endothelial cells derived from the aorta compared to that in the endothelium obtained from the coronary and iliac arteries. There were no statistically significant differences in the production of MMP-2 among the endothelial cell lines tested. The addition of κ-elastin at all evaluated concentrations did not statistically significantly influence the concentration of MMP-1 in the cultured coronary artery endothelium. Furthermore, no statistically significant differences were observed in the cultured iliac artery endothelium. In the cultured endothelium derived from the aorta, κ-elastin at concentrations of 0.1 and 0.4 μg/ml significantly increased the amount of MMP-1.
Figures

References
-
- Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function and biochemistry. Circ Res. 2003;92:827–839. - PubMed
-
- Nagase H, Woessner JF. Matrix metalloproteases. J Biol Chem. 1999;274:21491–21494. - PubMed
-
- Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;340:115–126. - PubMed
-
- Palombo D, Maione M, Cifiello BI, Udini M, Maggio D, Lupo M. Matrix metalloproteinases. Their role in degenerative chronic diseases of abdominal aorta. J Cardiovasc Surg. 1999;40:257–260. - PubMed
-
- Pearce WH, Shively VP. Abdominal aortic aneurysm as a complex multifactorial disease: interactions of polymorphisms of inflammatory genes, features of autoimmunity, and current status of MMPs. Ann NY Acad Sci. 2006;1085:117–132. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
