Chemical Diversity of Metabolites from Fungi, Cyanobacteria, and Plants Relative to FDA-Approved Anticancer Agents
- PMID: 22993669
- PMCID: PMC3443637
- DOI: 10.1021/ml300105s
Chemical Diversity of Metabolites from Fungi, Cyanobacteria, and Plants Relative to FDA-Approved Anticancer Agents
Abstract
A collaborative project has been undertaken to explore filamentous fungi, cyanobacteria, and tropical plants for anti-cancer drug leads. Through principal component analysis, the chemical space covered by compounds isolated and characterized from these three sources over the last four years was compared to each other and to the chemical space of selected FDA-approved anticancer drugs. Using literature precedence, nine molecular descriptors were examined: molecular weight, number of chiral centers, number of rotatable bonds, number of acceptor atoms for H-bonds (N,O,F), number of donor atoms for H-bonds (N and O), topological polar surface area using N,O polar contributions, Moriguchi octanol-water partition coefficient, number of nitrogen atoms, and number of oxygen atoms. Four principal components explained 87% of the variation found among 343 bioactive natural products and 96 FDA-approved anticancer drugs. Across the four dimensions, fungal, cyanobacterial and plant isolates occupied both similar and distinct areas of chemical space that collectively aligned well with FDA-approved anticancer agents. Thus, examining three separate re-sources for anticancer drug leads yields compounds that probe chemical space in a complementary fashion.
Figures
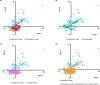
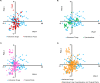
Similar articles
-
Discovery of Anticancer Agents of Diverse Natural Origin.Anticancer Res. 2016 Nov;36(11):5623-5637. doi: 10.21873/anticanres.11146. Anticancer Res. 2016. PMID: 27793884 Free PMC article. Review.
-
Characterization of Drug-like Chemical Space for Cytotoxic Marine Metabolites Using Multivariate Methods.ACS Omega. 2019 Mar 31;4(3):5402-5411. doi: 10.1021/acsomega.8b01764. Epub 2019 Mar 18. ACS Omega. 2019. PMID: 31179404 Free PMC article.
-
Chemoinformatic expedition of the chemical space of fungal products.Future Med Chem. 2016 Aug;8(12):1399-412. doi: 10.4155/fmc-2016-0079. Epub 2016 Aug 3. Future Med Chem. 2016. PMID: 27485744 Free PMC article.
-
Prioritization of anti-malarial hits from nature: chemo-informatic profiling of natural products with in vitro antiplasmodial activities and currently registered anti-malarial drugs.Malar J. 2016 Jan 29;15:50. doi: 10.1186/s12936-016-1087-y. Malar J. 2016. PMID: 26823078 Free PMC article.
-
Fungal endophytes as prolific source of phytochemicals and other bioactive natural products: A review.Microb Pathog. 2015 May;82:50-9. doi: 10.1016/j.micpath.2015.04.001. Epub 2015 Apr 9. Microb Pathog. 2015. PMID: 25865953 Review.
Cited by
-
Waol A, trans-dihydrowaol A, and cis-dihydrowaol A: polyketide-derived γ-lactones from a Volutella species.Tetrahedron Lett. 2013 Aug 7;54(32):4300-4302. doi: 10.1016/j.tetlet.2013.06.008. Tetrahedron Lett. 2013. PMID: 23956472 Free PMC article.
-
Studies on the epipolythiodioxopiperazine alkaloid verticillin D: Scaled production, streamlined purification, and absolute configuration.Phytochemistry. 2025 Aug;236:114492. doi: 10.1016/j.phytochem.2025.114492. Epub 2025 Mar 25. Phytochemistry. 2025. PMID: 40147592 Free PMC article.
-
Investigation of Vietnamese plants for potential anticancer agents.Phytochem Rev. 2014 Dec;13(4):727-739. doi: 10.1007/s11101-014-9335-7. Phytochem Rev. 2014. PMID: 25395897 Free PMC article.
-
Spiroscytalin, a new tetramic acid and other metabolites of mixed biogenesis from Scytalidium cuboideum.Tetrahedron. 2015 Nov 25;71(47):8899-904. doi: 10.1016/j.tet.2015.09.073. Tetrahedron. 2015. PMID: 26525642 Free PMC article.
-
Discovery of Anticancer Agents of Diverse Natural Origin.Anticancer Res. 2016 Nov;36(11):5623-5637. doi: 10.21873/anticanres.11146. Anticancer Res. 2016. PMID: 27793884 Free PMC article. Review.
References
-
- Kinghorn A. D.; Carcache de Blanco E. J.; Chai H. B.; Orjala J.; Farnsworth N. R.; Soejarto D. D.; Oberlies N. H.; Wani M. C.; Kroll D. J.; Pearce C. J.; Swanson S. M.; Kramer R. A.; Rose W. C.; Fairchild C. R.; Vite G. D.; Emanuel S.; Jarjoura D.; Cope F. O. Discovery of Anticancer Agents of Diverse Natural Origin. Pure Appl. Chem. 2009, 81, 1051–1063. - PMC - PubMed
-
- Orjala J.; Oberlies N. H.; Pearce C. J.; Swanson S. M.; Kinghorn A. D.. Discovery of Potential Anticancer Agents from Aquatic Cyanobacteria, Filamentous Fungi, and Tropical Plants. In Bioactive Compounds from Natural Sources. Natural Products as Lead Compounds in Drug Discovery, 2nd ed.; Tringali C., Ed.; Taylor & Francis: London, United Kingdom, 2012; pp 37–63.
-
- Ayers S.; Graf T. N.; Adcock A. F.; Kroll D. J.; Matthew S.; Carcache de Blanco E. J.; Shen Q.; Swanson S. M.; Wani M. C.; Pearce C. J.; Oberlies N. H. Resorcylic Acid Lactones with Cytotoxic and NF-kappaB Inhibitory Activities and Their Structure-Activity Relationships. J. Nat. Prod. 2011, 74, 1126–1131. - PMC - PubMed
-
- Ayers S.; Graf T. N.; Adcock A. F.; Kroll D. J.; Shen Q.; Swanson S. M.; Matthew S.; Carcache de Blanco E. J.; Wani M. C.; Darveaux B. A.; Pearce C. J.; Oberlies N. H. Cytotoxic Xanthone-Anthraquinone Heterodimers from an Unidentified Fungus of the Order Hypocreales (MSX 17022). J. Antibiot. 2012, 65, 3–8. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

