Aerobic exercise reduces oxidative stress and improves vascular changes of small mesenteric and coronary arteries in hypertension
- PMID: 22994554
- PMCID: PMC3579288
- DOI: 10.1111/j.1476-5381.2012.02224.x
Aerobic exercise reduces oxidative stress and improves vascular changes of small mesenteric and coronary arteries in hypertension
Abstract
Background and purpose: Regular physical activity is an effective non-pharmacological therapy for prevention and control of hypertension. We investigated the effects of aerobic exercise training in vascular remodelling and in the mechanical and functional alterations of coronary and small mesenteric arteries from spontaneously hypertensive rats (SHR).
Experimental approach: Normotensive Wistar Kyoto (WKY), SHR and SHR trained on a treadmill for 12 weeks were used to evaluate vascular structural, mechanical and functional properties.
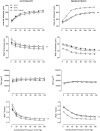
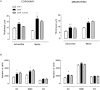
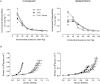
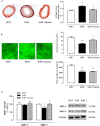
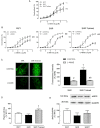
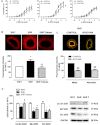
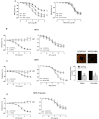
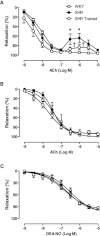
Key results: Exercise did not affect lumen diameter, wall thickness and wall/lumen ratio but reduced vascular stiffness of coronary and mesenteric arteries from SHR. Exercise also reduced collagen deposition and normalized altered internal elastic lamina organization and expression of MMP-9 in mesenteric arteries from SHR. Exercise did not affect contractile responses of coronary arteries but improved the endothelium-dependent relaxation in SHR. In mesenteric arteries, training normalized the increased contractile responses induced by U46619 and by high concentrations of acetylcholine. In vessels from SHR, exercise normalized the effects of the NADPH oxidase inhibitor apocynin and the NOS inhibitor l-NAME in vasodilator or vasoconstrictor responses, normalized the increased O(2) (-) production and the reduced Cu/Zn superoxide dismutase expression and increased NO production.
Conclusions and implications: Exercise training of SHR improves endothelial function and vascular stiffness in coronary and small mesenteric arteries. This might be related to the concomitant decrease of oxidative stress and increase of NO bioavailability. Such effects demonstrate the beneficial effects of exercise on the vascular system and could contribute to a reduction in blood pressure.
© 2012 The Authors. British Journal of Pharmacology © 2012 The British Pharmacological Society.
Figures
Similar articles
-
The elastic modulus of conductance coronary arteries from spontaneously hypertensive rats is increased.J Hypertens. 1997 Oct;15(10):1113-21. doi: 10.1097/00004872-199715100-00009. J Hypertens. 1997. PMID: 9350585
-
Liver growth factor treatment restores cell-extracellular matrix balance in resistance arteries and improves left ventricular hypertrophy in SHR.Am J Physiol Heart Circ Physiol. 2011 Sep;301(3):H1153-65. doi: 10.1152/ajpheart.00886.2010. Epub 2011 Jun 3. Am J Physiol Heart Circ Physiol. 2011. PMID: 21642499
-
Impact of moderate- and high-intensity exercise on the endothelial ultrastructure and function in mesenteric arteries from hypertensive rats.Life Sci. 2019 Apr 1;222:36-45. doi: 10.1016/j.lfs.2019.01.058. Epub 2019 Feb 27. Life Sci. 2019. PMID: 30825543 Free PMC article.
-
Cardiac changes in spontaneously hypertensive rats: Modulation by aerobic exercise.Prog Biophys Mol Biol. 2023 Jan;177:109-124. doi: 10.1016/j.pbiomolbio.2022.11.001. Epub 2022 Nov 5. Prog Biophys Mol Biol. 2023. PMID: 36347337 Review.
-
Edward F. Adolph Distinguished Lecture: The remarkable anti-aging effects of aerobic exercise on systemic arteries.J Appl Physiol (1985). 2014 Sep 1;117(5):425-39. doi: 10.1152/japplphysiol.00362.2014. Epub 2014 May 22. J Appl Physiol (1985). 2014. PMID: 24855137 Free PMC article. Review.
Cited by
-
Voluntary Wheel Running Attenuates Salt-Induced Vascular Stiffness Independent of Blood Pressure.Am J Hypertens. 2019 Nov 15;32(12):1162-1169. doi: 10.1093/ajh/hpz128. Am J Hypertens. 2019. PMID: 31401651 Free PMC article.
-
Pathophysiological mechanisms underlying the beneficial effects of physical activity in hypertension.J Clin Hypertens (Greenwich). 2020 Feb;22(2):291-295. doi: 10.1111/jch.13804. Epub 2020 Jan 19. J Clin Hypertens (Greenwich). 2020. PMID: 31955526 Free PMC article. No abstract available.
-
Supplementing Best Care with Specialized Rehabilitation Treatment in Parkinson's Disease: A Retrospective Study by Different Expert Centers.J Clin Med. 2024 May 20;13(10):2999. doi: 10.3390/jcm13102999. J Clin Med. 2024. PMID: 38792540 Free PMC article.
-
Aerobic Swim Training Restores Aortic Endothelial Function by Decreasing Superoxide Levels in Spontaneously Hypertensive Rats.Clinics (Sao Paulo). 2017 May;72(5):310-316. doi: 10.6061/clinics/2017(05)09. Clinics (Sao Paulo). 2017. PMID: 28591344 Free PMC article.
-
Voluntary wheel running prevents salt-induced endothelial dysfunction: role of oxidative stress.J Appl Physiol (1985). 2019 Feb 1;126(2):502-510. doi: 10.1152/japplphysiol.00421.2018. Epub 2018 Dec 20. J Appl Physiol (1985). 2019. PMID: 30571282 Free PMC article.
References
-
- Amaral SL, Zorn TMT, Michelini L. Exercise training normalizes wall-to-lumen ratio of the gracilis muscle arterioles and reduces pressure in spontaneously hypertensive rats. J Hypertens. 2000;18:1563–1572. - PubMed
-
- Arribas SM, Hillier C, González C, McGrory S, Dominiczak AF, McGrath JC. Cellular aspects of vascular remodeling in hypertension revealed by confocal microscopy. Hypertension. 1997;30:1455–1464. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

