Scope of the two-step, one-pot palladium-catalyzed borylation/Suzuki cross-coupling reaction utilizing bis-boronic acid
- PMID: 22994557
- PMCID: PMC3465529
- DOI: 10.1021/jo301642v
Scope of the two-step, one-pot palladium-catalyzed borylation/Suzuki cross-coupling reaction utilizing bis-boronic acid
Abstract
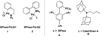
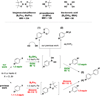
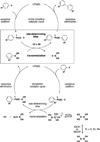
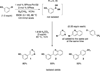
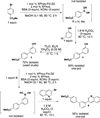
The use of bis-boronic acid for the direct synthesis of boronic acids has greatly facilitated the two-step, one-pot borylation/Suzuki cross-coupling reaction between aryl and heteroaryl halides. With use of Buchwald's second-generation XPhos preformed catalyst, high yields of cross-coupled products were obtained for most substrates. The method also allows an efficient two-step, one-pot synthesis, providing access to three distinct cross-coupled products after column chromatography. The method also provides a rapid and convenient route to teraryl compounds.
Figures

References
-
- Bellina F, Carpita A, Rossi R. Synthesis. 2004:2419–2440.
-
- Kotha S, Lahiri K, Kashinath D. Tetrahedron. 2002;58:9633–9695.
-
- Miyaura N, Yanagi T, Suzuki A. Synth. Commun. 1981:513–519.
-
- Suzuki A. Acc. Chem. Res. 1982;15:178–184.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

