Differing effects of rapamycin or calcineurin inhibitor on T-regulatory cells in pediatric liver and kidney transplant recipients
- PMID: 22994804
- PMCID: PMC3513508
- DOI: 10.1111/j.1600-6143.2012.04269.x
Differing effects of rapamycin or calcineurin inhibitor on T-regulatory cells in pediatric liver and kidney transplant recipients
Abstract
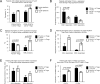
In a cross-sectional study, we assessed effects of calcineurin inhibitor (CNI) or rapamycin on T-regulatory (Treg) cells from children with stable liver (n = 53) or kidney (n = 9) allografts several years posttransplant. We analyzed Treg number, phenotype, suppressive function, and methylation at the Treg-specific demethylation region (TSDR) using Tregs and peripheral blood mononuclear cells. Forty-eight patients received CNI (39 as monotherapy) and 12 patients received rapamycin (9 as monotherapy). Treg numbers diminished over time on either regimen, but reached significance only with CNI (r =-0.424, p = 0.017). CNI levels inversely correlated with Treg number (r =-0.371, p = 0.026), and positively correlated with CD127+ expression by Tregs (r = 0.437, p = 0.023). Patients with CNI levels >3.6 ng/mL had weaker Treg function than those with levels <3.6 ng/mL, whereas rapamycin therapy positively correlated with Treg numbers (r = 0.628, p = 0.029) and their expression of CTLA4 (r = 0.726, p = 0.041). Overall, CTLA4 expression, TSDR demethylation and an absence of CD127 were important for Treg suppressive function. We conclude that rapamycin has beneficial effects on Treg biology, whereas long-term and high dose CNI use may impair Treg number, function and phenotype, potentially acting as a barrier to attaining host hyporesponsiveness to an allograft.
© Copyright 2012 The American Society of Transplantation and the American Society of Transplant Surgeons.
Figures



References
-
- Srinivas TR, Kaplan B. Transplantation in 2011: New agents, new ideas and new hope. Nature reviews Nephrology. 2012;8:74–75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

