Characterization of anti-HIV-1 neutralizing and binding antibodies in chronic HIV-1 subtype C infection
- PMID: 22995189
- PMCID: PMC3488441
- DOI: 10.1016/j.virol.2012.08.033
Characterization of anti-HIV-1 neutralizing and binding antibodies in chronic HIV-1 subtype C infection
Abstract
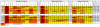
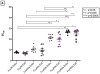
Neutralizing (nAbs) and high affinity binding antibodies may be critical for an efficacious HIV-1 vaccine. We characterized virus-specific nAbs and binding antibody responses over 21 months in eight HIV-1 subtype C chronically infected individuals with heterogeneous rates of disease progression. Autologous nAb titers of study exit plasma against study entry viruses were significantly higher than contemporaneous responses at study entry (p=0.002) and exit (p=0.01). NAb breadth and potencies against subtype C viruses were significantly higher than for subtype A (p=0.03 and p=0.01) or B viruses (p=0.03; p=0.05) respectively. Gp41-IgG binding affinity was higher than gp120-IgG (p=0.0002). IgG-FcγR1 affinity was significantly higher than FcγRIIIa (p<0.005) at study entry and FcγRIIb (p<0.05) or FcγRIIIa (p<0.005) at study exit. Evolving IgG binding suggests alteration of immune function mediated by binding antibodies. Evolution of nAbs was a potential marker of HIV-1 disease progression.
Copyright © 2012 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures





References
-
- Allain JP, Laurian Y, Paul DA, Verroust F, Leuther M, Gazengel C, Senn D, Larrieu MJ, Bosser C. Long-term evaluation of HIV antigen and antibodies to p24 and gp41 in patients with hemophilia. Potential clinical importance. N Engl J Med. 1987;317:1114–1121. - PubMed
-
- Baum LL, Cassutt KJ, Knigge K, Khattri R, Margolick J, Rinaldo C, Kleeberger CA, Nishanian P, Henrard DR, Phair J. HIV-1 gp120-specific antibody-dependent cell-mediated cytotoxicity correlates with rate of disease progression. J Immunol. 1996;157:2168–2173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

