Characterization of cellular furin content as a potential factor determining the susceptibility of cultured human and animal cells to coronavirus infectious bronchitis virus infection
- PMID: 22995191
- PMCID: PMC7111921
- DOI: 10.1016/j.virol.2012.08.037
Characterization of cellular furin content as a potential factor determining the susceptibility of cultured human and animal cells to coronavirus infectious bronchitis virus infection
Abstract
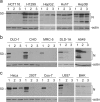
In previous studies, the Beaudette strain of coronavirus infectious bronchitis virus (IBV) was adapted from chicken embryo to Vero, a monkey kidney cell line, by serial propagation for 65 passages. To characterize the susceptibility of other human and animal cells to IBV, 15 human and animal cell lines were infected with the Vero-adapted IBV and productive infection was observed in four human cell lines: H1299, HepG2, Hep3B and Huh7. In other cell lines, the virus cannot be propagated beyond passage 5. Interestingly, cellular furin abundance in five human cell lines was shown to be strongly correlated with productive IBV infection. Cleavage of IBV spike protein by furin may contribute to the productive IBV infection in these cells. The findings that IBV could productively infect multiple human and animal cells of diverse tissue and organ origins would provide a useful system for studying the pathogenesis of coronavirus.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Treatment with Exogenous Trypsin Expands In Vitro Cellular Tropism of the Avian Coronavirus Infectious Bronchitis Virus.Viruses. 2020 Sep 29;12(10):1102. doi: 10.3390/v12101102. Viruses. 2020. PMID: 33003350 Free PMC article.
-
Proteolytic activation of the spike protein at a novel RRRR/S motif is implicated in furin-dependent entry, syncytium formation, and infectivity of coronavirus infectious bronchitis virus in cultured cells.J Virol. 2009 Sep;83(17):8744-58. doi: 10.1128/JVI.00613-09. Epub 2009 Jun 24. J Virol. 2009. PMID: 19553314 Free PMC article.
-
The S2 Subunit of Infectious Bronchitis Virus Beaudette Is a Determinant of Cellular Tropism.J Virol. 2018 Sep 12;92(19):e01044-18. doi: 10.1128/JVI.01044-18. Print 2018 Oct 1. J Virol. 2018. PMID: 30021894 Free PMC article.
-
Avian infectious bronchitis virus.Rev Sci Tech. 2000 Aug;19(2):493-508. doi: 10.20506/rst.19.2.1228. Rev Sci Tech. 2000. PMID: 10935276 Review.
-
Recent progress in studies of arterivirus- and coronavirus-host interactions.Viruses. 2012 Jun;4(6):980-1010. doi: 10.3390/v4060980. Epub 2012 Jun 19. Viruses. 2012. PMID: 22816036 Free PMC article. Review.
Cited by
-
Infectious Bronchitis Virus Infection Induces Apoptosis during Replication in Chicken Macrophage HD11 Cells.Viruses. 2017 Jul 26;9(8):198. doi: 10.3390/v9080198. Viruses. 2017. PMID: 28933760 Free PMC article.
-
Treatment with Exogenous Trypsin Expands In Vitro Cellular Tropism of the Avian Coronavirus Infectious Bronchitis Virus.Viruses. 2020 Sep 29;12(10):1102. doi: 10.3390/v12101102. Viruses. 2020. PMID: 33003350 Free PMC article.
-
Avian Coronavirus Infectious Bronchitis Virus Activates Mitochondria-Mediated Apoptosis Pathway and Affects Viral Replication by Inducing Reactive Oxygen Species Production in Chicken HD11 Cells.Biology (Basel). 2024 Jul 1;13(7):491. doi: 10.3390/biology13070491. Biology (Basel). 2024. PMID: 39056685 Free PMC article.
-
Identification of two ATR-dependent phosphorylation sites on coronavirus nucleocapsid protein with nonessential functions in viral replication and infectivity in cultured cells.Virology. 2013 Sep;444(1-2):225-32. doi: 10.1016/j.virol.2013.06.014. Epub 2013 Jul 9. Virology. 2013. PMID: 23849791 Free PMC article.
-
Natural selection differences detected in key protein domains between non-pathogenic and pathogenic Feline Coronavirus phenotypes.bioRxiv [Preprint]. 2023 Jan 11:2023.01.11.523607. doi: 10.1101/2023.01.11.523607. bioRxiv. 2023. Update in: Virus Evol. 2023 Mar 15;9(1):vead019. doi: 10.1093/ve/vead019. PMID: 36712007 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

