Microfluidic chips with reversed-phase monoliths for solid phase extraction and on-chip labeling
- PMID: 22995197
- PMCID: PMC3463737
- DOI: 10.1016/j.chroma.2012.08.095
Microfluidic chips with reversed-phase monoliths for solid phase extraction and on-chip labeling
Abstract
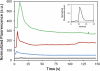
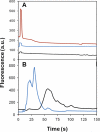
The integration of sample preparation methods into microfluidic devices provides automation necessary for achieving complete micro total analysis systems. We have developed a technique that combines on-chip sample enrichment with fluorescence labeling and purification. Polymer monoliths made from butyl methacrylate were fabricated in cyclic olefin copolymer microdevices and used for solid phase extraction. We studied the retention of fluorophores, amino acids and proteins on these columns. The retained samples were subsequently labeled with both Alexa Fluor 488 and Chromeo P503, and unreacted dye was rinsed off the column before sample elution. Additional purification was obtained from the differential retention of proteins and fluorescent labels. A linear relation between the eluted peak areas and concentrations of on-chip labeled heat shock protein 90 samples demonstrated the utility of this method for on-chip quantitation. Our fast and simple method of simultaneously concentrating and labeling samples on-chip is compatible with miniaturization and desirable for automated analysis.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures





Similar articles
-
On-Chip Fluorescent Labeling using Reversed-phase Monoliths and Microchip Electrophoretic Separations of Selected Preterm Birth Biomarkers.Anal Methods. 2016 Nov 21;8(43):7739-7746. doi: 10.1039/C6AY01803C. Epub 2016 Sep 30. Anal Methods. 2016. PMID: 28496521 Free PMC article.
-
Automated microfluidic devices integrating solid-phase extraction, fluorescent labeling, and microchip electrophoresis for preterm birth biomarker analysis.Anal Bioanal Chem. 2018 Jan;410(3):933-941. doi: 10.1007/s00216-017-0548-7. Epub 2017 Aug 10. Anal Bioanal Chem. 2018. PMID: 28799040 Free PMC article.
-
Integrated electrokinetically driven microfluidic devices with pH-mediated solid-phase extraction coupled to microchip electrophoresis for preterm birth biomarkers.Electrophoresis. 2017 Jul;38(13-14):1743-1754. doi: 10.1002/elps.201700054. Epub 2017 Apr 25. Electrophoresis. 2017. PMID: 28272749 Free PMC article.
-
Advances in coupling microfluidic chips to mass spectrometry.Mass Spectrom Rev. 2015 Sep-Oct;34(5):535-57. doi: 10.1002/mas.21417. Epub 2014 Jan 7. Mass Spectrom Rev. 2015. PMID: 24399782 Review.
-
Monolithic methacrylate packed 96-tips for high throughput bioanalysis.J Chromatogr A. 2010 Apr 16;1217(16):2581-8. doi: 10.1016/j.chroma.2009.10.072. Epub 2009 Oct 31. J Chromatogr A. 2010. PMID: 19931088 Review.
Cited by
-
Microfluidics for Peptidomics, Proteomics, and Cell Analysis.Nanomaterials (Basel). 2021 Apr 26;11(5):1118. doi: 10.3390/nano11051118. Nanomaterials (Basel). 2021. PMID: 33925983 Free PMC article. Review.
-
Development of an integrated microfluidic solid-phase extraction and electrophoresis device.Analyst. 2016 Mar 7;141(5):1660-8. doi: 10.1039/c5an02352a. Analyst. 2016. PMID: 26820409 Free PMC article.
-
Monitoring cell secretions on microfluidic chips using solid-phase extraction with mass spectrometry.Anal Bioanal Chem. 2017 Jan;409(1):169-178. doi: 10.1007/s00216-016-9983-0. Epub 2016 Oct 19. Anal Bioanal Chem. 2017. PMID: 27761614 Free PMC article.
-
Advances in microfluidic materials, functions, integration, and applications.Chem Rev. 2013 Apr 10;113(4):2550-83. doi: 10.1021/cr300337x. Epub 2013 Feb 14. Chem Rev. 2013. PMID: 23410114 Free PMC article. Review. No abstract available.
-
3D Printed Microfluidic Devices for Integrated Immunoaffinity Extraction, Solid-Phase Extraction, and Fluorescent Labeling of Preterm Birth Biomarkers.Precis Chem. 2025 Mar 3;3(5):261-271. doi: 10.1021/prechem.4c00092. eCollection 2025 May 26. Precis Chem. 2025. PMID: 40443764 Free PMC article.
References
-
- Angelescu DE. Highly Integrated Microfluidics Design. Artech House. 2011
-
- Yang H, Mudrik JM, Jebrail MJ, Wheeler AR. Anal. Chem. 2011;83:3824. - PubMed
-
- Hagan KA, Reedy CR, Bienvenue JM, Dewald AH, Landers JP. Analyst. 2011;136:1928. - PubMed
-
- Hua Y, Jemere AB, Harrison DJ. J. Chromatogr. A. 2011;1218:4039. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

