Expression of the vitamin D-activating enzyme 1α-hydroxylase (CYP27B1) decreases during melanoma progression
- PMID: 22995334
- PMCID: PMC3529817
- DOI: 10.1016/j.humpath.2012.03.031
Expression of the vitamin D-activating enzyme 1α-hydroxylase (CYP27B1) decreases during melanoma progression
Abstract
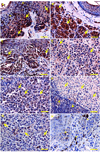
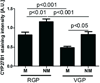
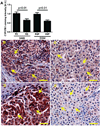
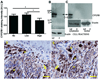
1α-Hydroxylase (CYP27B1), the enzyme responsible for the synthesis of the biologically active form of vitamin D (1,25(OH)(2)D(3)), is expressed in the skin. To assess the correlation between progression of melanocytic tumors and CYP27B1, we analyzed its expression in 29 benign nevi, 75 primary cutaneous melanomas, 40 metastases, and 4 re-excision and 6 normal skin biopsies. Immunoreactivity for CYP27B1 was significantly lower in the vertical growth phase and metastatic melanomas (0.6 and 0.5 arbitrary units, respectively) in comparison with nevi and radial growth phase tumors (1.2 and 1.1 arbitrary units, respectively); and expression was reduced in more advanced lesions (Clark levels III-V, Breslow thickness ≥2.1 mm; 0.8 and 0.7 arbitrary units, respectively). There was an inverse correlation between CYP27B1 and Ki-67 expression. Furthermore, CYP27B1 expression was reduced in primary melanomas that created metastases in comparison with non-metastasizing melanomas. Reduced CYP27B1 expression in radial growth phase was related to shorter overall survival (810 versus 982 versus 1151 days in melanomas with absent, low, and high CYP27B1 immunoreactivity), and low CYP27B1 expression in radial growth phase and vertical growth phase was related to shorter disease-free survival (114 versus 339 versus 737 days and 129 versus 307 versus 737 days, respectively, in melanomas with absent, low, and high CYP27B1). Also, CYP27B1 expression was inversely related to melanin in melanoma cells in vivo and melanoma cells cultured in vitro. Thus, reduction of CYP27B1 correlates with melanoma phenotype and behavior, and its lack affects the survival of melanoma patients, indicating a role in the pathogenesis and progression of this cancer.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
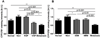
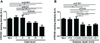

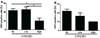

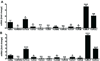
References
-
- Wang TT, Tavera-Mendoza LE, Laperriere D, Libby E, MacLeod NB, Nagai Y, et al. Large-scale in silico and microarray-based identification of direct 1,25-dihydroxyvitamin D3 target genes. Mol Endocrinol. 2005;19:2685–2695. - PubMed
-
- Chen H, Hu B, Allegretto EA, Adams JS. The vitamin D response element-binding protein: a novel dominant-negative regulator of vitamin D-directed transactivation. J Biol Chem. 2000;275:35557–35564. - PubMed
-
- Colston K, Colston MJ, Feldman D. 1,25-Dihydroxyvitamin D3 and malignant melanoma: the presence of receptors and inhibition of cell growth in culture. Endocrinology. 1981;108:1083–1086. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

