Cellular response to heat shock studied by multiconfocal fluorescence correlation spectroscopy
- PMID: 22995483
- PMCID: PMC3446677
- DOI: 10.1016/j.bpj.2012.07.041
Cellular response to heat shock studied by multiconfocal fluorescence correlation spectroscopy
Abstract
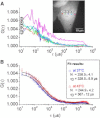
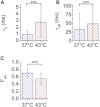
Heat shock triggers a transient and ubiquitous response, the function of which is to protect cells against stress-induced damage. The heat-shock response is controlled by a key transcription factor known as heat shock factor 1 (HSF1). We have developed a multiconfocal fluorescence correlation spectroscopy setup to measure the dynamics of HSF1 during the course of the heat-shock response. The system combines a spatial light modulator, to address several points of interest, and an electron-multiplying charge-coupled camera for fast multiconfocal recording of the photon streams. Autocorrelation curves with a temporal resolution of 14 μs were analyzed before and after heat shock on eGFP and HSF1-eGFP-expressing cells. Evaluation of the dynamic parameters of a diffusion-and-binding model showed a slower HSF1 diffusion after heat shock. It is also observed that the dissociation rate decreases after heat shock, whereas the association rate is not affected. In addition, thanks to the multiconfocal fluorescence correlation spectroscopy system, up to five spots could be simultaneously located in each cell nucleus. This made it possible to quantify the intracellular variability of the diffusion constant of HSF1, which is higher than that of inert eGFP molecules and increases after heat shock. This finding is consistent with the fact that heat-shock response is associated with an increase of HSF1 interactions with DNA and cannot be explained even partially by heat-induced modifications of nuclear organization.
Copyright © 2012 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Dynamics of the full length and mutated heat shock factor 1 in human cells.PLoS One. 2013 Jul 8;8(7):e67566. doi: 10.1371/journal.pone.0067566. Print 2013. PLoS One. 2013. PMID: 23861773 Free PMC article.
-
Multi-confocal fluorescence correlation spectroscopy.Front Biosci (Elite Ed). 2011 Jan 1;3(2):476-88. doi: 10.2741/e263. Front Biosci (Elite Ed). 2011. PMID: 21196328
-
Heat shock-induced interactions among nuclear HSFs detected by fluorescence cross-correlation spectroscopy.Biochem Biophys Res Commun. 2015 Jul 31;463(3):303-8. doi: 10.1016/j.bbrc.2015.05.056. Epub 2015 May 21. Biochem Biophys Res Commun. 2015. PMID: 26003728
-
Interactions between extracellular signal-regulated protein kinase 1, 14-3-3epsilon, and heat shock factor 1 during stress.J Biol Chem. 2004 Nov 19;279(47):49460-9. doi: 10.1074/jbc.M406059200. Epub 2004 Sep 10. J Biol Chem. 2004. PMID: 15364926
-
Stress-induced activation of the heat-shock response: cell and molecular biology of heat-shock factors.Biochem Soc Symp. 1999;64:105-18. Biochem Soc Symp. 1999. PMID: 10207624 Review.
Cited by
-
48-spot single-molecule FRET setup with periodic acceptor excitation.J Chem Phys. 2018 Mar 28;148(12):123304. doi: 10.1063/1.5000742. J Chem Phys. 2018. PMID: 29604810 Free PMC article.
-
Quantitative imaging of mammalian transcriptional dynamics: from single cells to whole embryos.BMC Biol. 2016 Dec 23;14(1):115. doi: 10.1186/s12915-016-0331-9. BMC Biol. 2016. PMID: 28010727 Free PMC article. Review.
-
Estimating binding properties of transcription factors from genome-wide binding profiles.Nucleic Acids Res. 2015 Jan;43(1):84-94. doi: 10.1093/nar/gku1269. Epub 2014 Nov 28. Nucleic Acids Res. 2015. PMID: 25432957 Free PMC article.
-
Genome-wide off-rates reveal how DNA binding dynamics shape transcription factor function.Mol Syst Biol. 2020 Oct;16(10):e9885. doi: 10.15252/msb.20209885. Mol Syst Biol. 2020. PMID: 33280256 Free PMC article.
-
Dual functions of the ZmCCT-associated quantitative trait locus in flowering and stress responses under long-day conditions.BMC Plant Biol. 2016 Nov 3;16(1):239. doi: 10.1186/s12870-016-0930-1. BMC Plant Biol. 2016. PMID: 27809780 Free PMC article.
References
-
- Wiseman P.W., Brown C.M., Horwitz A.F. Spatial mapping of integrin interactions and dynamics during cell migration by image correlation microscopy. J. Cell Sci. 2004;117:5521–5534. - PubMed

