K⁺ conduction and Mg²⁺ blockade in a shaker Kv-channel single point mutant with an unusually high conductance
- PMID: 22995492
- PMCID: PMC3446664
- DOI: 10.1016/j.bpj.2012.08.015
K⁺ conduction and Mg²⁺ blockade in a shaker Kv-channel single point mutant with an unusually high conductance
Abstract
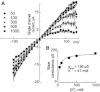
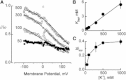
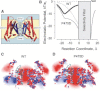
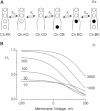
Potassium channels exhibit a large diversity of single-channel conductances. Shaker is a low-conductance K-channel in which Pro475→Asp, a single-point mutation near the internal pore entrance, promotes 6- to 8-fold higher unitary current. To assess the mechanism for this higher conductance, we measured Shaker-P475D single-channel current in a wide range of symmetrical K(+) concentrations and voltages. Below 300 mM K(+), the current-to-voltage relations (i-V) showed inward rectification that disappeared at 1000 mM K(+). Single-channel conductance reached a maximum of ∼190 pS at saturating [K(+)], a value 4- to 5-fold larger than that estimated for the native channel. Intracellular Mg(2+) blocked this variant with ∼100-fold higher affinity. Near zero voltage, blockade was competitively antagonized by K(+); however, at voltages >100 mV, it was enhanced by K(+). This result is consistent with a lock-in effect in a single-file diffusion regime of Mg(2+) and K(+) along the pore. Molecular-dynamics simulations revealed higher K(+) density in the pore, especially near the Asp-475 side chains, as in the high-conductance MthK bacterial channel. The molecular dynamics also showed that K(+) ions bound distally can coexist with other K(+) or Mg(2+) in the cavity, supporting a lock-in mechanism. The maximal K(+) transport rate and higher occupancy could be due to a decrease in the electrostatic energy profile for K(+) throughout the pore, reducing the energy wells and barriers differentially by ∼0.7 and ∼2 kT, respectively.
Copyright © 2012 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures





Similar articles
-
Pore dimensions and the role of occupancy in unitary conductance of Shaker K channels.J Gen Physiol. 2015 Aug;146(2):133-46. doi: 10.1085/jgp.201411353. J Gen Physiol. 2015. PMID: 26216859 Free PMC article.
-
An extracellular Cu2+ binding site in the voltage sensor of BK and Shaker potassium channels.J Gen Physiol. 2008 May;131(5):483-502. doi: 10.1085/jgp.200809980. J Gen Physiol. 2008. PMID: 18443360 Free PMC article.
-
Multi-ion distributions in the cytoplasmic domain of inward rectifier potassium channels.Biophys J. 2012 Aug 8;103(3):434-443. doi: 10.1016/j.bpj.2012.06.023. Biophys J. 2012. PMID: 22947859 Free PMC article.
-
Mechanism of rectification in inward-rectifier K+ channels.Annu Rev Physiol. 2004;66:103-29. doi: 10.1146/annurev.physiol.66.032102.150822. Annu Rev Physiol. 2004. PMID: 14977398 Review.
-
Involvement of the S4-S5 linker and the C-linker domain regions to voltage-gating in plant Shaker channels: comparison with animal HCN and Kv channels.Plant Signal Behav. 2014;9(10):e972892. doi: 10.4161/15592316.2014.972892. Plant Signal Behav. 2014. PMID: 25482770 Free PMC article. Review.
Cited by
-
Hydrophobic interaction between contiguous residues in the S6 transmembrane segment acts as a stimuli integration node in the BK channel.J Gen Physiol. 2015 Jan;145(1):61-74. doi: 10.1085/jgp.201411194. J Gen Physiol. 2015. PMID: 25548136 Free PMC article.
-
What keeps Kv channels small? The molecular physiology of modesty.J Gen Physiol. 2015 Aug;146(2):123-7. doi: 10.1085/jgp.201511469. J Gen Physiol. 2015. PMID: 26216857 Free PMC article. No abstract available.
-
Initial steps of inactivation at the K+ channel selectivity filter.Proc Natl Acad Sci U S A. 2014 Apr 29;111(17):E1713-22. doi: 10.1073/pnas.1317573111. Epub 2014 Apr 14. Proc Natl Acad Sci U S A. 2014. PMID: 24733889 Free PMC article.
-
Nernst equilibrium, rectification, and saturation: Insights into ion channel behavior.Biophys J. 2024 Dec 17;123(24):4304-4315. doi: 10.1016/j.bpj.2024.10.016. Epub 2024 Oct 30. Biophys J. 2024. PMID: 39478342
-
Multiscale Responsive Kinetic Modeling: Quantifying Biomolecular Reaction Flux under Varying Electrochemical Conditions.bioRxiv [Preprint]. 2024 Aug 2:2024.08.01.606205. doi: 10.1101/2024.08.01.606205. bioRxiv. 2024. Update in: J Chem Theory Comput. 2025 Jan 14;21(1):374-389. doi: 10.1021/acs.jctc.4c00872. PMID: 39131358 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

