How lipid headgroups sense the membrane environment: an application of ¹⁴N NMR
- PMID: 22995497
- PMCID: PMC3446661
- DOI: 10.1016/j.bpj.2012.08.031
How lipid headgroups sense the membrane environment: an application of ¹⁴N NMR
Abstract
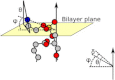
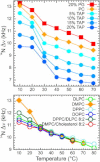
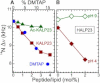
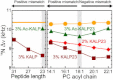
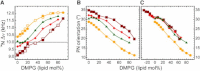
The orientation of lipid headgroups may serve as a powerful sensor of electrostatic interactions in membranes. As shown previously by (2)H NMR measurements, the headgroup of phosphatidylcholine (PC) behaves like an electrometer and varies its orientation according to the membrane surface charge. Here, we explored the use of solid-state (14)N NMR as a relatively simple and label-free method to study the orientation of the PC headgroup in model membrane systems of varying composition. We found that (14)N NMR is sufficiently sensitive to detect small changes in headgroup orientation upon introduction of positively and negatively charged lipids and we developed an approach to directly convert the (14)N quadrupolar splittings into an average orientation of the PC polar headgroup. Our results show that inclusion of cholesterol or mixing of lipids with different length acyl chains does not significantly affect the orientation of the PC headgroup. In contrast, measurements with cationic (KALP), neutral (Ac-KALP), and pH-sensitive (HALP) transmembrane peptides show very systematic changes in headgroup orientation, depending on the amount of charge in the peptide side chains and on their precise localization at the interface, as modulated by varying the extent of hydrophobic peptide/lipid mismatch. Finally, our measurements suggest an unexpectedly strong preferential enrichment of the anionic lipid phosphatidylglycerol around the cationic KALP peptide in ternary mixtures with PC. We believe that these results are important for understanding protein/lipid interactions and that they may help parametrization of membrane properties in computational studies.
Copyright © 2012 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
PMP1 18-38, a yeast plasma membrane protein fragment, binds phosphatidylserine from bilayer mixtures with phosphatidylcholine: a (2)H-NMR study.Biophys J. 2000 Nov;79(5):2624-31. doi: 10.1016/S0006-3495(00)76501-5. Biophys J. 2000. PMID: 11053135 Free PMC article.
-
The cytoplasmic domains of phospholamban and phospholemman associate with phospholipid membrane surfaces.Biochemistry. 2005 Dec 27;44(51):17016-26. doi: 10.1021/bi0511383. Biochemistry. 2005. PMID: 16363815
-
Human opioid peptide Met-enkephalin binds to anionic phosphatidylserine in high preference to zwitterionic phosphatidylcholine: natural-abundance 13C NMR study on the binding state in large unilamellar vesicles.Biochemistry. 2006 Dec 26;45(51):15601-9. doi: 10.1021/bi061641v. Biochemistry. 2006. PMID: 17176081
-
Role of interactions at the lipid-water interface for domain formation.Mol Membr Biol. 1995 Jan-Mar;12(1):83-8. doi: 10.3109/09687689509038500. Mol Membr Biol. 1995. PMID: 7767388
-
How lipids affect the activities of integral membrane proteins.Biochim Biophys Acta. 2004 Nov 3;1666(1-2):62-87. doi: 10.1016/j.bbamem.2004.05.012. Biochim Biophys Acta. 2004. PMID: 15519309 Review.
Cited by
-
Tuning the Extracellular Vesicles Membrane through Fusion for Biomedical Applications.J Funct Biomater. 2023 Feb 19;14(2):117. doi: 10.3390/jfb14020117. J Funct Biomater. 2023. PMID: 36826916 Free PMC article.
-
An efficient NMR method for the characterisation of 14N sites through indirect 13C detection.Phys Chem Chem Phys. 2013 May 28;15(20):7613-20. doi: 10.1039/c3cp50787d. Epub 2013 Apr 15. Phys Chem Chem Phys. 2013. PMID: 23589073 Free PMC article.
-
Multiscale Dynamics of Lipid Vesicles in Polymeric Microenvironment.Membranes (Basel). 2022 Jun 21;12(7):640. doi: 10.3390/membranes12070640. Membranes (Basel). 2022. PMID: 35877843 Free PMC article.
-
Lipid Head Group Parameterization for GROMOS 54A8: A Consistent Approach with Protein Force Field Description.J Chem Theory Comput. 2019 Oct 8;15(10):5175-5193. doi: 10.1021/acs.jctc.9b00509. Epub 2019 Sep 9. J Chem Theory Comput. 2019. PMID: 31433640 Free PMC article.
-
C60 fullerene localization and membrane interactions in RAW 264.7 immortalized mouse macrophages.Nanoscale. 2016 Feb 21;8(7):4134-44. doi: 10.1039/c5nr07003a. Nanoscale. 2016. PMID: 26866469 Free PMC article.
References
-
- Seelig J., Macdonald P.M., Scherer P.G. Phospholipid head groups as sensors of electric charge in membranes. Biochemistry. 1987;26:7535–7541. - PubMed
-
- Jensen M.Ø., Mouritsen O.G. Lipids do influence protein function-the hydrophobic matching hypothesis revisited. Biochim. Biophys. Acta. 2004;1666:205–226. - PubMed
-
- Poolman B., Spitzer J.J., Wood J.M. Bacterial osmosensing: roles of membrane structure and electrostatics in lipid-protein and protein-protein interactions. Biochim. Biophys. Acta. 2004;1666:88–104. - PubMed
-
- Lee A.G. How lipids affect the activities of integral membrane proteins. Biochim. Biophys. Acta. 2004;1666:62–87. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

