How lipid headgroups sense the membrane environment: an application of ¹⁴N NMR
- PMID: 22995497
- PMCID: PMC3446661
- DOI: 10.1016/j.bpj.2012.08.031
How lipid headgroups sense the membrane environment: an application of ¹⁴N NMR
Abstract
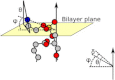
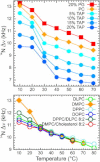
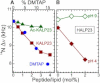
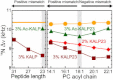
The orientation of lipid headgroups may serve as a powerful sensor of electrostatic interactions in membranes. As shown previously by (2)H NMR measurements, the headgroup of phosphatidylcholine (PC) behaves like an electrometer and varies its orientation according to the membrane surface charge. Here, we explored the use of solid-state (14)N NMR as a relatively simple and label-free method to study the orientation of the PC headgroup in model membrane systems of varying composition. We found that (14)N NMR is sufficiently sensitive to detect small changes in headgroup orientation upon introduction of positively and negatively charged lipids and we developed an approach to directly convert the (14)N quadrupolar splittings into an average orientation of the PC polar headgroup. Our results show that inclusion of cholesterol or mixing of lipids with different length acyl chains does not significantly affect the orientation of the PC headgroup. In contrast, measurements with cationic (KALP), neutral (Ac-KALP), and pH-sensitive (HALP) transmembrane peptides show very systematic changes in headgroup orientation, depending on the amount of charge in the peptide side chains and on their precise localization at the interface, as modulated by varying the extent of hydrophobic peptide/lipid mismatch. Finally, our measurements suggest an unexpectedly strong preferential enrichment of the anionic lipid phosphatidylglycerol around the cationic KALP peptide in ternary mixtures with PC. We believe that these results are important for understanding protein/lipid interactions and that they may help parametrization of membrane properties in computational studies.
Copyright © 2012 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Seelig J., Macdonald P.M., Scherer P.G. Phospholipid head groups as sensors of electric charge in membranes. Biochemistry. 1987;26:7535–7541. - PubMed
-
- Jensen M.Ø., Mouritsen O.G. Lipids do influence protein function-the hydrophobic matching hypothesis revisited. Biochim. Biophys. Acta. 2004;1666:205–226. - PubMed
-
- Poolman B., Spitzer J.J., Wood J.M. Bacterial osmosensing: roles of membrane structure and electrostatics in lipid-protein and protein-protein interactions. Biochim. Biophys. Acta. 2004;1666:88–104. - PubMed
-
- Lee A.G. How lipids affect the activities of integral membrane proteins. Biochim. Biophys. Acta. 2004;1666:62–87. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

