Kinetic mechanism of Ca²⁺-controlled changes of skeletal troponin I in psoas myofibrils
- PMID: 22995498
- PMCID: PMC3446660
- DOI: 10.1016/j.bpj.2012.08.022
Kinetic mechanism of Ca²⁺-controlled changes of skeletal troponin I in psoas myofibrils
Abstract
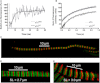
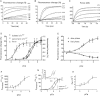
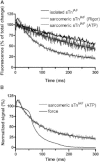
Conformational changes in the skeletal troponin complex (sTn) induced by rapidly increasing or decreasing the [Ca(2+)] were probed by 5-iodoacetamidofluorescein covalently bound to Cys-133 of skeletal troponin I (sTnI). Kinetics of conformational changes was determined for the isolated complex and after incorporating the complex into rabbit psoas myofibrils. Isolated and incorporated sTn exhibited biphasic Ca(2+)-activation kinetics. Whereas the fast phase (k(obs)∼1000 s(-1)) is only observed in this study, where kinetics were induced by Ca(2+), the slower phase resembles the monophasic kinetics of sTnI switching observed in another study (Brenner and Chalovich. 1999. Biophys. J. 77:2692-2708) that investigated the sTnI switching induced by releasing the feedback of force-generating cross-bridges on thin filament activation. Therefore, the slower conformational change likely reflects the sTnI switch that regulates force development. Modeling reveals that the fast conformational change can occur after the first Ca(2+) ion binds to skeletal troponin C (sTnC), whereas the slower change requires Ca(2+) binding to both regulatory sites of sTnC. Incorporating sTn into myofibrils increased the off-rate and lowered the Ca(2+) sensitivity of sTnI switching. Comparison of switch-off kinetics with myofibril force relaxation kinetics measured in a mechanical setup indicates that sTnI switching might limit the rate of fast skeletal muscle relaxation.
Copyright © 2012 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Lys184 deletion in troponin I impairs relaxation kinetics and induces hypercontractility in murine cardiac myofibrils.Cardiovasc Res. 2008 Mar 1;77(4):676-86. doi: 10.1093/cvr/cvm113. Epub 2007 Dec 20. Cardiovasc Res. 2008. PMID: 18096573
-
Kinetic mechanism of the Ca2+-dependent switch-on and switch-off of cardiac troponin in myofibrils.Biophys J. 2007 Dec 1;93(11):3917-31. doi: 10.1529/biophysj.107.111146. Epub 2007 Aug 17. Biophys J. 2007. PMID: 17704185 Free PMC article.
-
Thin filament Ca2+ binding properties and regulatory unit interactions alter kinetics of tension development and relaxation in rabbit skeletal muscle.J Physiol. 2008 Aug 1;586(15):3683-700. doi: 10.1113/jphysiol.2008.152181. Epub 2008 Jun 5. J Physiol. 2008. PMID: 18535094 Free PMC article.
-
Sarcomeric determinants of striated muscle relaxation kinetics.Pflugers Arch. 2005 Mar;449(6):505-17. doi: 10.1007/s00424-004-1363-5. Epub 2004 Nov 30. Pflugers Arch. 2005. PMID: 15750836 Review.
-
Mechanism of cross-bridge detachment in isometric force relaxation of skeletal and cardiac myofibrils.J Muscle Res Cell Motil. 2003;24(4-6):261-7. J Muscle Res Cell Motil. 2003. PMID: 14620739 Review.
Cited by
-
Assessing Cardiac Contractility From Single Molecules to Whole Hearts.JACC Basic Transl Sci. 2023 Oct 11;9(3):414-439. doi: 10.1016/j.jacbts.2023.07.013. eCollection 2024 Mar. JACC Basic Transl Sci. 2023. PMID: 38559627 Free PMC article. Review.
-
Kinetic coupling of phosphate release, force generation and rate-limiting steps in the cross-bridge cycle.J Muscle Res Cell Motil. 2017 Aug;38(3-4):275-289. doi: 10.1007/s10974-017-9482-8. Epub 2017 Sep 16. J Muscle Res Cell Motil. 2017. PMID: 28918606 Review.
-
Cycling Cross-Bridges Contribute to Thin Filament Activation in Human Slow-Twitch Fibers.Front Physiol. 2020 Mar 24;11:144. doi: 10.3389/fphys.2020.00144. eCollection 2020. Front Physiol. 2020. PMID: 32265723 Free PMC article.
-
Structural dynamics of troponin during activation of skeletal muscle.Proc Natl Acad Sci U S A. 2014 Mar 25;111(12):4626-31. doi: 10.1073/pnas.1321868111. Epub 2014 Mar 10. Proc Natl Acad Sci U S A. 2014. PMID: 24616505 Free PMC article.
-
The dilated cardiomyopathy-causing mutation ACTC E361G in cardiac muscle myofibrils specifically abolishes modulation of Ca(2+) regulation by phosphorylation of troponin I.Biophys J. 2014 Nov 18;107(10):2369-80. doi: 10.1016/j.bpj.2014.10.024. Biophys J. 2014. PMID: 25418306 Free PMC article.
References
-
- Farah C.S., Reinach F.C. The troponin complex and regulation of muscle contraction. FASEB J. 1995;9:755–767. - PubMed
-
- Gordon A.M., Homsher E., Regnier M. Regulation of contraction in striated muscle. Physiol. Rev. 2000;80:853–924. - PubMed
-
- Rosenfeld S.S., Taylor E.W. Kinetic studies of calcium and magnesium binding to troponin C. J. Biol. Chem. 1985;260:242–251. - PubMed
-
- Herzberg O., Moult J., James M.N. A model for the Ca2+-induced conformational transition of troponin C. A trigger for muscle contraction. J. Biol. Chem. 1986;261:2638–2644. - PubMed
-
- Dong W., Rosenfeld S.S., Cheung H.C. Kinetic studies of calcium binding to the regulatory site of troponin C from cardiac muscle. J. Biol. Chem. 1996;271:688–694. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

