Multi-sensory integration in brainstem and auditory cortex
- PMID: 22995545
- PMCID: PMC3489970
- DOI: 10.1016/j.brainres.2012.08.037
Multi-sensory integration in brainstem and auditory cortex
Abstract
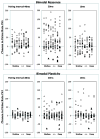
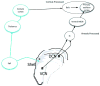
Tinnitus is the perception of sound in the absence of a physical sound stimulus. It is thought to arise from aberrant neural activity within central auditory pathways that may be influenced by multiple brain centers, including the somatosensory system. Auditory-somatosensory (bimodal) integration occurs in the dorsal cochlear nucleus (DCN), where electrical activation of somatosensory regions alters pyramidal cell spike timing and rates of sound stimuli. Moreover, in conditions of tinnitus, bimodal integration in DCN is enhanced, producing greater spontaneous and sound-driven neural activity, which are neural correlates of tinnitus. In primary auditory cortex (A1), a similar auditory-somatosensory integration has been described in the normal system (Lakatos et al., 2007), where sub-threshold multisensory modulation may be a direct reflection of subcortical multisensory responses (Tyll et al., 2011). The present work utilized simultaneous recordings from both DCN and A1 to directly compare bimodal integration across these separate brain stations of the intact auditory pathway. Four-shank, 32-channel electrodes were placed in DCN and A1 to simultaneously record tone-evoked unit activity in the presence and absence of spinal trigeminal nucleus (Sp5) electrical activation. Bimodal stimulation led to long-lasting facilitation or suppression of single and multi-unit responses to subsequent sound in both DCN and A1. Immediate (bimodal response) and long-lasting (bimodal plasticity) effects of Sp5-tone stimulation were facilitation or suppression of tone-evoked firing rates in DCN and A1 at all Sp5-tone pairing intervals (10, 20, and 40 ms), and greater suppression at 20 ms pairing-intervals for single unit responses. Understanding the complex relationships between DCN and A1 bimodal processing in the normal animal provides the basis for studying its disruption in hearing loss and tinnitus models. This article is part of a Special Issue entitled: Tinnitus Neuroscience.
Published by Elsevier B.V.
Figures


References
-
- Cappe C, Barone P. Heteromodal connections supporting multisensory integration at low levels of cortical processing in the monkey. Eur J Neurosci. 2005;22:2886–2902. - PubMed
-
- Davis KA, Miller RL, Young ED. Effects of somatosensory and parallel-fiber stimulation on neurons in dorsal cochlear nucleus. J Neurophysiol. 1996;76:3012–3024. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

