Is the mitochondrial outermembrane protein VDAC1 therapeutic target for Alzheimer's disease?
- PMID: 22995655
- PMCID: PMC3518645
- DOI: 10.1016/j.bbadis.2012.09.003
Is the mitochondrial outermembrane protein VDAC1 therapeutic target for Alzheimer's disease?
Abstract
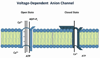
Mitochondrial dysfunction and synaptic damage have been described as early events in Alzheimer's disease (AD) pathogenesis. Recent research using AD postmortem brains, and AD mouse and cell models revealed that amyloid beta (Aβ) and tau hyperphosphorylation are involved in mitochondrial dysfunction and synaptic damage in AD. Further, recent research also revealed that the protein levels of mitochondrial outer membrane protein, voltage-dependent anion channel 1 (VDAC1), are elevated in the affected regions of AD postmortem brains and cortical tissues from APP transgenic mice. In addition, emerging research using AD postmortem brains and AD mouse models revealed that VDAC1 is linked to Aβ and phosphorylated tau, blocks the mitochondrial permeability transition (MPT) pores, disrupts the transport of mitochondrial proteins and metabolites, impairs gating of VDAC, and causes defects in oxidative phosphorylation, leading to mitochondrial dysfunction in AD neurons. The purpose of this article is to review research that has investigated the relationship between VDAC1 and the regulation of MPT pores in AD progression.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures


Similar articles
-
Abnormal interaction of VDAC1 with amyloid beta and phosphorylated tau causes mitochondrial dysfunction in Alzheimer's disease.Hum Mol Genet. 2012 Dec 1;21(23):5131-46. doi: 10.1093/hmg/dds360. Epub 2012 Aug 27. Hum Mol Genet. 2012. PMID: 22926141 Free PMC article.
-
The Voltage-dependent Anion Channel 1 Mediates Amyloid β Toxicity and Represents a Potential Target for Alzheimer Disease Therapy.J Biol Chem. 2015 Dec 25;290(52):30670-83. doi: 10.1074/jbc.M115.691493. Epub 2015 Nov 5. J Biol Chem. 2015. PMID: 26542804 Free PMC article.
-
VDAC1, mitochondrial dysfunction, and Alzheimer's disease.Pharmacol Res. 2018 May;131:87-101. doi: 10.1016/j.phrs.2018.03.010. Epub 2018 Mar 15. Pharmacol Res. 2018. PMID: 29551631 Review.
-
RNA silencing of genes involved in Alzheimer's disease enhances mitochondrial function and synaptic activity.Biochim Biophys Acta. 2013 Dec;1832(12):2368-78. doi: 10.1016/j.bbadis.2013.09.008. Epub 2013 Sep 21. Biochim Biophys Acta. 2013. PMID: 24063855 Free PMC article.
-
Amyloid beta-induced glycogen synthase kinase 3β phosphorylated VDAC1 in Alzheimer's disease: implications for synaptic dysfunction and neuronal damage.Biochim Biophys Acta. 2013 Dec;1832(12):1913-21. doi: 10.1016/j.bbadis.2013.06.012. Epub 2013 Jun 28. Biochim Biophys Acta. 2013. PMID: 23816568 Free PMC article. Review.
Cited by
-
Use and Reuse of Animal Behavioral, Molecular, and Biochemical Data in Alzheimer's Disease Research: Focus on 3Rs and Saving People's Tax Dollars.J Alzheimers Dis Rep. 2024 Sep 3;8(1):1171-1184. doi: 10.3233/ADR-240126. eCollection 2024. J Alzheimers Dis Rep. 2024. PMID: 39247873 Free PMC article. Review.
-
A Mitocentric View of Alzheimer's Disease.Mol Neurobiol. 2017 Oct;54(8):6046-6060. doi: 10.1007/s12035-016-0117-7. Epub 2016 Oct 1. Mol Neurobiol. 2017. PMID: 27696116 Review.
-
PHD-2 activation: a novel strategy to control HIF-1α and mitochondrial stress to modulate mammary gland pathophysiology in ER+ subtype.Naunyn Schmiedebergs Arch Pharmacol. 2019 Oct;392(10):1239-1256. doi: 10.1007/s00210-019-01658-7. Epub 2019 Jun 1. Naunyn Schmiedebergs Arch Pharmacol. 2019. PMID: 31154466
-
Voltage-dependent anion channels mediated apoptosis in refractory epilepsy.Open Med (Wars). 2020 Aug 3;15(1):745-753. doi: 10.1515/med-2020-0113. eCollection 2020. Open Med (Wars). 2020. PMID: 33336032 Free PMC article.
-
Accumulation of Basic Amino Acids at Mitochondria Dictates the Cytotoxicity of Aberrant Ubiquitin.Cell Rep. 2015 Mar 10;10(9):1557-1571. doi: 10.1016/j.celrep.2015.02.009. Epub 2015 Mar 5. Cell Rep. 2015. PMID: 25753421 Free PMC article.
References
-
- Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol. Rev. 2001;81:741–766. - PubMed
-
- Alzheimer Association, Alzheimer’s disease: Facts and Figures. 2012.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

