Histone deacetylase inhibitor (HDACi) suberoylanilide hydroxamic acid (SAHA)-mediated correction of α1-antitrypsin deficiency
- PMID: 22995909
- PMCID: PMC3488095
- DOI: 10.1074/jbc.M112.404707
Histone deacetylase inhibitor (HDACi) suberoylanilide hydroxamic acid (SAHA)-mediated correction of α1-antitrypsin deficiency
Abstract
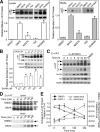
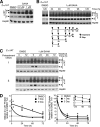
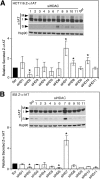
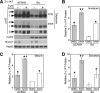
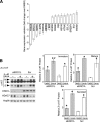
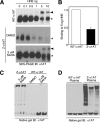
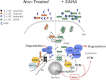
α1-Antitrypsin (α1AT) deficiency (α1ATD) is a consequence of defective folding, trafficking, and secretion of α1AT in response to a defect in its interaction with the endoplasmic reticulum proteostasis machineries. The most common and severe form of α1ATD is caused by the Z-variant and is characterized by the accumulation of α1AT polymers in the endoplasmic reticulum of the liver leading to a severe reduction (>85%) of α1AT in the serum and its anti-protease activity in the lung. In this organ α1AT is critical for ensuring tissue integrity by inhibiting neutrophil elastase, a protease that degrades elastin. Given the limited therapeutic options in α1ATD, a more detailed understanding of the folding and trafficking biology governing α1AT biogenesis and its response to small molecule regulators is required. Herein we report the correction of Z-α1AT secretion in response to treatment with the histone deacetylase (HDAC) inhibitor suberoylanilide hydroxamic acid (SAHA), acting in part through HDAC7 silencing and involving a calnexin-sensitive mechanism. SAHA-mediated correction restores Z-α1AT secretion and serpin activity to a level 50% that observed for wild-type α1AT. These data suggest that HDAC activity can influence Z-α1AT protein traffic and that SAHA may represent a potential therapeutic approach for α1ATD and other protein misfolding diseases.
Figures

References
-
- Balch W. E., Morimoto R. I., Dillin A., Kelly J. W. (2008) Adapting proteostasis for disease intervention. Science 319, 916–919 - PubMed
-
- Powers E. T., Morimoto R. I., Dillin A., Kelly J. W., Balch W. E. (2009) Biological and chemical approaches to diseases of proteostasis deficiency. Annu. Rev. Biochem. 78, 959–991 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

